Deepak Chowrasia* and Nisha Sharma
University Institute of Pharmacy, CSJM University, Kanpur-208024, India
*Corresponding Author:
Deepak Chowrasia
University Institute of Pharmacy
CSJM University
Kanpur-208024, India
Tel: +91-9451-019049
E-mail: chowrasia.deepak@gmail.com
Received date: November 02, 2016; Accepted date: November 11, 2016; Published date: November 18, 2016
Citation: Chowrasia D, Sharma N. Solvent Substitution Evaluation of Limestone Water as a Medium for Benzoylation. Arch Chem Res. 2016, 1:1. doi: 10.21767/2572-4657.100001
Keywords
Benzoylation; Limestone water; Industrial technique; Solvent substitution
Introduction
“No coopora nisi fluida”, the terms depicts extent and need of solvent for a chemical reaction to proceed, was coined by Greece Philosopher Aristotle. However, it has now been found that reactions to an extent may occur even in solventless environment where host reactant and attacking reagent directly comes in contact with each other. Both the techniques when compared in terms of synthetic scenario have their own pros and cons nevertheless use of solvent to assist a chemical reaction is still unquestionable [1] owing to their decisive direct influence on outcomes such as reaction rate, product purity, its yield, economic value, and eco-friendly technique. Currently, a paradigm shift in chemical manufacturing industry has now been noticed prominently in the field of solvent substitution where manufacturing processes are shifted from conventional hazardous to newer less hazardous solvfents without compromising their synthetic practicability including net manufacturing cost however management of later is still an industrial challenging task.
Benzoylation (introduction of ArCO- group; Scheme 1) is an effective, economic, and handy technique to protect and identify amino as well as hydroxyl functionality present in an aromatic as well as aliphatic organic compounds including their synthetic chemical transformation subsequently into amide (Ar’CONHAr/R) or ester derivatives (Ar’CO-OAr/R) in the presence of alkaline solvents such as aqueous solution of sodium hydroxide or pyridine [2-6] along with benzoylating agent-benzoyl chloride or their substituted derivatives (Schotten-Baumann reaction) [7,8]. High melting point, resistance towards hydrolysis in aqueous medium, and insolubility of benzoylated derivatives makes the technique of benzoylation advantageously distinguished among acetylation (introduction of RCO-group), thus preferred comparatively [7,8].
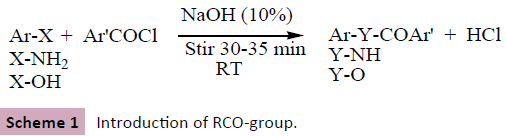
Scheme 1: Introduction of RCO-group.
Materials and Methods
All the reagents and solvents (Figure 1) used in this experimentation are acquired from common store University Institute of Pharmacy, CSJM University, Kanpur, India and used as and their basis without any modification unless or until specified. The limestone used for preparation of water for benzoylation was purchased from Kidwai Nagar marble market, Kanpur, India and was used unmodified. Since compounds synthesised to evaluate limestone water for elucidating its feasibility for benzoylation are reported and spectrally characterized hence a comparative study between synthesized and reported compounds were done to enumerate its practicability and data regarding same is reported in Table 1 including supplementary file uploaded herewith. The melting point for synthesised compounds was recorded by open capillary method in triplicate and is uncorrected. The progression of reaction was monitored in PET ether:ethylacetate (8:2) as a binary solvent system.
| Host reactants |
Benzoylating agent |
Solvent |
Product |
Reaction time (min.) |
Melting point (ºC)
R F |
Yield (%) |
| Phenol |
Benzoyl chloride |
Limestone water |
Phenyl benzoate (1a) |
13 |
70 72 |
52 |
| Aniline |
Benzoyl chloride |
Limestone water |
Benzanilide (1b) |
4 |
162 161 |
74 |
| 1-naphthol |
Benzoyl chloride |
Limestone water |
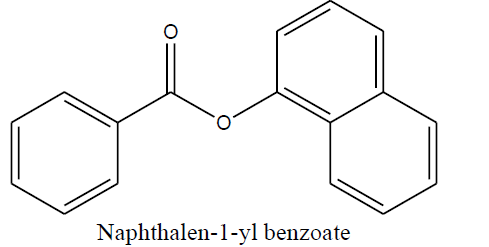
Naphthalen-1-yl benzoate (1c) |
27 |
56 57 |
43 |
| 2-naphthol |
Benzoyl chloride |
Limestone water |
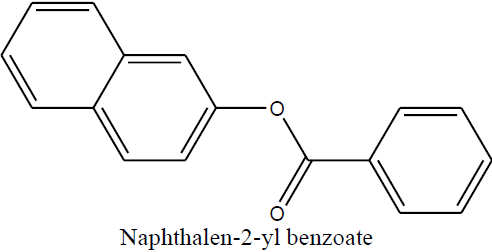
Naphthalen-2-yl benzoate (1d) |
19 |
107 106 |
63 |
| 4-hydroxy acetanilide |
Benzoyl chloride |
Limestone water |
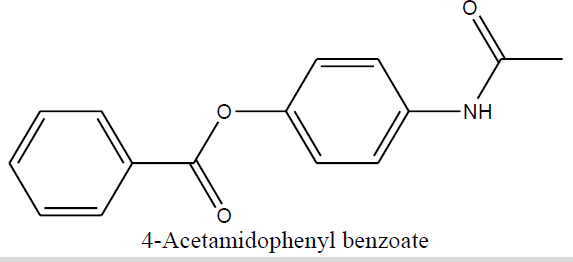
4-Acetamidophenyl benzoate (1e) |
9 |
? 201 |
70 |
| Vanillin |
Benzoyl chloride |
Limestone water |
4-Benzoyloxy-3-methoxy benzaldehyde (1f) |
6* |
77 75 |
38 |
| Resorcinol |
Benzoyl chloride |
Limestone water |
3-hydroxy phenyl benzoate (1g) |
22 |
135 133 |
55 |
Table 1 Generalized description of synthesized compounds (*days, R-Reported, F-Found).

Figure 1: Limestone (left) and a child holding limestone water (Right).
Experimental
The efficiency of limestone water as a medium for synthesizing benzoylated derivatives was evaluated by dissolving or suspending equimolar quantity (0.01 M) of reactants and benzoyl chloride in limestone water. The content was shaken for the sufficient period of time, yielding crude product, was further washed thoroughly from cold water and finally recrystallized from ethanol.
Note
Equimolar quantities of reactants and benzoyl chloride (slightly in excess amount and dropwise) was transferred in a well cleaned conical flask under the fuming hood. The flask was corked securely and resultant mixture vigorously shaken at room temperature until the product precipitate out from solution or odour of benzoyl chloride cease to evolve. In case if not precipitated, the flask was immersed in ice-chest or kept aside at room temperature until product is obtained. The crude product is filtered off and washed thoroughly with cold water followed by a biphasic mixture containing equal quantity of water and methanol. Once dried properly the same get recrystallized initially from water:methanol (1:1) and finally from ethanol.
Results and Discussion
The solvent substitute technique for benzoylation we herein develops and reported is unique and advantageous over conventional benzoylation methodology in terms of cost effectiveness, eco-compatibility, and is entirely free from using any harmful strong basic medium. The limestone water was successfully evaluated to elucidate its synthetic harmony for benzoylating monocarbocyclic as well as polycarbocyclic substituted and unsubstituted aromatic compounds containing hydroxyl and amino functionality. We further claims that the technique we herein reported can equally be applied for yielding benzoylated derivatives of aliphatic compounds containing hydroxyl and amino groups including protecting N-terminal ends of amino acids for peptide synthesis however the same was reserved as a future workup plan of ours. Among synthesized compounds, 1b was yielded in greater amount comparatively followed by 1e, 1d, 1g, and 1a (Supplementary File). However compound 1f and 1c (Supplementary File) were obtained in smaller quantities. Furthermore, evaluating limestone water towards completion of reaction it was found that benzoylated derivatives obtained from mononuclear ring system was yielded sooner than those obtained from polynuclear ring system except 1g and 1f (Supplementary File). The overall mean reaction time for mono and polycyclic ring system was calculated and found to be 12 and 23-minutes respectively. However when calculated individually the compound 1b, 1d, and 1e (Supplementary File) were synthesized within a time interval of 10-minutes; 1b was the fasted yielded compound with an overall time duration of 4-minutes; else were synthesized within a time range of 13-22 minutes, 1f (Supplementary File) being the last to obtain (6 days).
To conclude the above phenomenon and get insight the differential reactivity pattern of these compounds structure oriented study was further included, was found that aromatic monocyclic ring system containing –OH/-NH (Tables 2 and 3) functionality was easily benzoylated compared to their polycyclic analogues this may be because of distribution of electrons among single and polynuclear ring system. However when compared among, ring containing -NH functionality reacts rapidly with higher yield of final product (1b) than those containing –OH group. This may be assumed due to difference in reactivity pattern of functional group with benzoylating agent-benzoyl chloride (Tables 4 and 5).
| Compounds |
Test |
Result |
Inferences |
| Phenyl benzoate (1a) |
Ferric chloride test |
Negative |
No free OH group present; benzoylation done |
| Benzanilide (1b) |
Ferric chloride test |
Not applicable |
Not applicable |
| Naphthalen-1-yl benzoate (1c) |
Ferric chloride test |
Negative |
No free OH group present; benzoylation done |
| Naphthalen-2-yl benzoate (1d) |
Ferric chloride test |
Negative |
No free OH group present; benzoylation done |
| 4-Acetamidophenyl benzoate (1e) |
Ferric chloride test |
Negative |
No free OH group present; benzoylation done |
| 4-Benzoyloxy-3-methoxy benzaldehyde (1f) |
Ferric chloride test |
Negative |
No free OH group present; benzoylation done |
| 3-hydroxy phenyl benzoate (1g) |
Ferric chloride test |
Violet color |
Single free OH group present; benzoylation done |
| Phenyl benzoate (1a) |
Phthalein test |
Negative |
No free OH group present; benzoylation done |
| Benzanilide (1b) |
Phthalein test |
Not applicable |
Not applicable |
| Naphthalen-1-yl benzoate (1c) |
Phthalein test |
Negative |
No free OH group present; benzoylation done |
| Naphthalen-2-yl benzoate (1d) |
Phthalein test |
Negative |
No free OH group present; benzoylation done |
| 4-Acetamidophenyl benzoate (1e) |
Phthalein test |
Negative |
No free OH group present; benzoylation done |
| 4-Benzoyloxy-3-methoxy benzaldehyde (1f) |
Phthalein test |
Negative |
No free OH group present; benzoylation done |
| 3-hydroxy phenyl benzoate (1g) |
Phthalein test |
Light green color |
Single free OH group present; benzoylation done |
Table 2 Qualitative test for presence of free –OH group in synthesized products.
| Compounds |
Test |
Result |
Inferences |
| Phenyl benzoate (1a) |
Dye test |
Not applicable |
Not applicable |
| Benzanilide (1b) |
Dye test |
Negative |
No free –NH2 group is present; benzoylation done |
| Naphthalen-1-yl benzoate (1c) |
Dye test |
Not applicable |
Not applicable |
| Naphthalen-2-yl benzoate (1d) |
Dye test |
Not applicable |
Not applicable |
| 4-Acetamidophenyl benzoate (1e) |
Dye test |
Not applicable |
Not applicable |
| 4-Benzoyloxy-3-methoxy benzaldehyde (1f) |
Dye test |
Not applicable |
Not applicable |
| 3-hydroxy phenyl benzoate (1g) |
Dye test |
Not applicable |
Not applicable |
| Phenyl benzoate (1a) |
Bleaching sol test |
Not applicable |
Not applicable |
| Benzanilide (1b) |
Bleaching sol test |
Negative |
No free –NH2 group is present; benzoylation done |
| Naphthalen-1-yl benzoate (1c) |
Bleaching sol test |
Not applicable |
Not applicable |
| Naphthalen-2-yl benzoate (1d) |
Bleaching sol test |
Not applicable |
Not applicable |
| 4-Acetamidophenyl benzoate (1e) |
Bleaching sol test |
Not applicable |
Not applicable |
| 4-Benzoyloxy-3-methoxy benzaldehyde (1f) |
Bleaching sol test |
Not applicable |
Not applicable |
| 3-hydroxy phenyl benzoate (1g) |
Bleaching sol test |
Not applicable |
Not applicable |
Table 3 Qualitative test for presence of free –NH group in synthesized products.
| Compounds |
Water |
NaOH
(10%) |
NaHCO3
(10%) |
Acetic acid(10%) |
HCl
(10%) |
Methanol
(50%) |
Ethanol
(100%) |
Chloroform
(100%) |
| Phenyl benzoate (1a) |
- |
+ |
+ |
-+ |
+ |
+ |
+++ |
+++ |
| Benzanilide (1b) |
- |
- |
- |
-+ |
+ |
++ |
+++ |
+++ |
| Naphthalen-1-yl benzoate (1c) |
- |
- |
- |
-+ |
-+ |
+ |
+++ |
+++ |
| Naphthalen-2-yl benzoate (1d) |
- |
- |
- |
-+ |
-+ |
+ |
+++ |
+++ |
| 4-Acetamidophenyl benzoate (1e) |
-+ |
-+ |
- |
-+ |
-+ |
++ |
+++ |
+++ |
| 4-Benzoyloxy-3-methoxy benzaldehyde (1f) |
- |
- |
- |
-+ |
-+ |
++ |
+++ |
+++ |
| 3-hydroxy phenyl benzoate (1g) |
-+ |
-+ |
- |
-+ |
-+ |
++ |
+++ |
+++ |
Table 4 Solubility profile of synthesized compounds in different solvents.
| Compounds |
Structure |
| Phenyl benzoate (1a) |
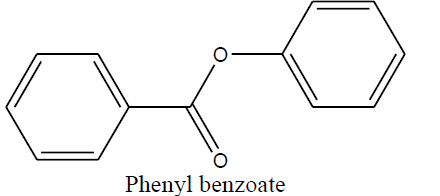 |
| Benzanilide |
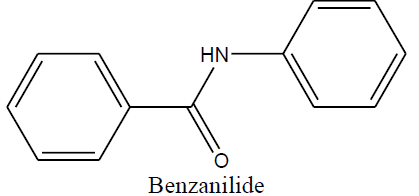 |
| Naphthalen-1-yl benzoate (1c) |
 |
| Naphthalen-2-yl benzoate (1d) |
 |
| 4-Acetamidophenyl benzoate (1e) |
 |
| 4-Benzoyloxy-3-methoxy benzaldehyde (1f) |
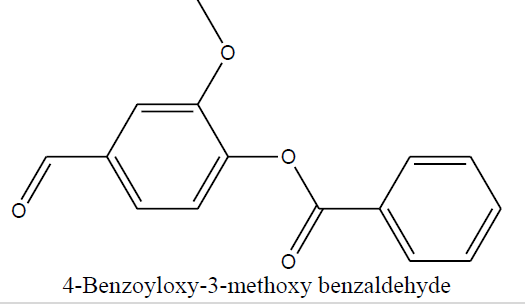 |
| 3-hydroxy phenyl benzoate (1g) |
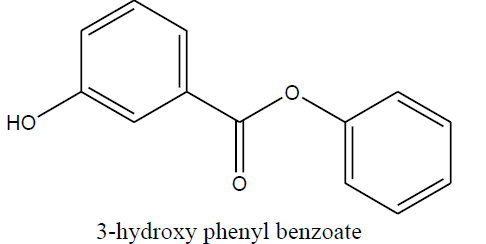 |
Table 5 Structure of synthesized compounds.
Conclusion
Though overall yield of benzoylated derivatives we found in limestone water is not comparable with traditional alkaline solvents nevertheless its practicability in terms of cheap and ecocompatible solvent is undeniable. Furthermore the limestone water if used along with other alkaline solvent the net cost for industrial production for yielding benzoylated derivatives could efficiently be minimized. Since limestone water is comparatively less basic than conventional alkaline solvents hence this technique could be used for benzoylation of amino acid without causing their racemization. However as per our prediction, the limestone water could be used efficiently for synthesis of compounds 1b and 1d (Supplementary File) and with substantial modification for compounds 1a, 1c, and 1g (Supplementary File).
References
- Tapia O,Bertran J (2002) Solvent effect and chemical reactivity. Kluwer academic publisher, Dordrecht p: 17.
- Greene TW (1981) Protective groups in organic synthesis, Wiley NY, USA pp:261-263.
- Reese CB (1973) Protective groups in organic chemistry, Plenum press London, UK pp: 52-53.
- Ando W,Tsumaki H (1983) A facile preparation of aliphatic hydroxamic acid from N,N,O-tris(trimethylsilyl)hydroxylamine and acid chloride. Synthetic communications 13: 1053-1056.
- Taylor EC, Mclay GW, McKillop A (1968) Thallium in organic synthesis. II. Acylation, Aroylation, and tosylation, of phenol and carboxylic acid.Journal of American chemical Society90: 2422-2423.
- Illi VO (1979) Phase transfer catalyzed acylation.Tetrahedron Letters 20: 2431-2432.
- Vogel AI (1956) Practical organic chemistry: Longman group limited London pp: 582-583.
- Mann FG, Saunders BS (1978) Practical organic chemistry, Longman group limited London pp:243-244.