Keywords
Nanostructure, Lipid, Quarcetin, Cancer, Nasal, Drug delivery.
Introduction
Brain tumours are intracranial lesions that occupy space in the skull. Brain tumours are, in fact, the second leading cause of cancer related deaths in children and young adults. Cancer is the most distressing and life threatening disease that enforces severe death worldwide [1]. The intra cranial lesions in the skull are known as brain tumours. It is the second leading cause of the cancer related deaths in children and young adults. Brain tumour is an abnormal growth occurring in any tissue contained within the cranium, including the brain, cranial nerves, meninges, skull, pituitary gland, and pineal gland [2]. For treatment of cancer, chemotherapy is widely used, but it has many limitations such as nonselective drug distribution, multi drug resistance, and increased drug toxicity to the normal cells [3]. The ultimate goal of cancer therapy is to increase the survival time of patient by targeted drug delivery that will ultimately reduce the toxicity of chemotherapy to normal cells [4]. In case of treatment of brain tumour the main aim is targeted delivery of some hydrophobic molecules bypassing the physical barrier of the CNS i.e., Blood Brain Barrier (BBB). Quercetin (QUE) is a nutritional molecule can be used to maintain the general health in healthy peoples and can be use as nutraceutical to cure disease. Various studies show that QUE processes active anti-inflammatory with anti-cancer activity. It is found in many fruits, vegetables, leaves and grains [5]. QUE exerts its more specific pro-apopticaction on tumour cells than non-transformedcells. Because of the hydrophobic and crystalline nature of QUE like molecules their direct addition in to food product is not possible thus the biological activity of such molecules is get reduce [6]. Although quercetin shows several beneficial actions, its anticancer activity is get hindered because of its low solubility in water. So to overcome the solubility problem of quercetin efforts has been taken for development of novel preparations that enhance solubility of QUE and increase its bioavailability in inhibition of tumour [7]. For the effective nose to brain delivery of the drug colloidal drug delivery is the promising approach for the delivery of hydrophobic drugs, as it has several advantages of increased bioavailability, Sustain release of the drug, and improved drug stability [8]. Because of the versatile nature of the lipids their uses in formulation of nanoparticles enhance activity of drug after oral administration [9]. Nano carriers have the potential for the targeted delivery of drug to tumour site. The use of NPs to deliver drugs to the brain across the BBB may provide a significant advantage over currently used strategies. NLC are colloidal carriers characterized by a solid lipid core consisting of a mixture of solid and liquid lipids, and having a mean particle size in the nanometre range. Their lipophilic features facilitate crossing the BBB to enter the brain by endocytosis. Several drugs have been incorporated into lipid NPs (NLCs), which are potentially useful for the treatment of brain diseases. Nanoparticles and their use in drug delivery is more effectual antitumor method than conventional chemotherapy which is typically limited by the toxicity of drugs to normal tissues, short circulation half-life in plasma, limited aqueous solubility, and non-selectivity restricting therapeutic efficacy [10]. The aim of present study was design of the NLC formulation of quercetin by taking in account effect different factors on formulation and evaluation of nanostructure lipid carriers of quercetin for intranasal delivery to CNS.
Materials and Methods
Materials
Quercetin was obtained from Otto chemie. Pvt. Ltd, Mumbai, India. Glyceryl monostearate was purchased from Merck Pvt Ltd., Mumbai, Capmul GMO was obtained from ABITEC Corporation (Columbus, OH), Polaxomer 188 was obtained from Hi-Media Lab. Pvt. Ltd., Mumbai, Soya lecithin Phospholipid was obtained from GmbH, Krefeld, Germany. All other reagents used were of analytical grade.
Formulation of QUE-NLCs
QUE-NLCs were formulated by using hot high pressure homogenisation (HPH) technique. Briefly, for the hot HPH method, the lipid phase containing Glyceryl mono stearate and capmul GMO was heated up to 85 ± 0.5°C to melt the lipids and QUE (10 mg/ml) was dispersed in molten lipids. Pre-emulsion was prepared by dispersing the hot aqueous surfactant (polaxomer188 and soya lecithin) solution to above melted lipid phase under mechanical stirring (Remi instruments Ltd, Mumbai, India) at 1500 rpm at 85 ± 0.5°C for 20 mins. This preemulsion was then passed through HPH (PANDA 2K, NiroSoavi, Italy) at 600 bar pressure and for 10 cycles. During processing of the samples, machine was coated with a heater tape and the temperature of the homogenization circuit was maintained at 85 ± 0.5°C to avoid risk of boiling. The resulting hot o/w Nano emulsion was cooled to 4 ± 0.5°C, recrystallization of the lipid and QUE loaded NLCs (QUE-NLCs) were generated. The QUE-NLCs’ aqueous dispersion was centrifuge at 45000 rpm for 35 mins at room temperature by using Optima ‘‘MAX-XP’’ ultracentrifuge (Beckman Coulter, Nyon, Switzerland) for the separation of the Nano-particulate system. Deposited particulate was redispersed in little amount of water. The QUE-NLCs’ aqueous dispersion with 5% cryoprotectant (mannitol) was frozen in a refrigerator at 70°C for 12 h. Then the samples were lyophilized using a lyophilizer (Vir-Tis Benchtop, SP Scientific, Warminster, PA) and maintained at 2-8°C [11].
Particle Size and Zeta Potential of QUE-NLCs
Particle size of NLC suspension was determined using a photon correlation spectrometer (Zeta sizer nano ZS 90, Malvern Ltd., UK) equipped with a 4.0 mW internal laser and based on the Dynamic Light Scattering phenomenon, while the zeta potential, reflecting the electric charge on the particle surface and indicating the physical stability of colloidal systems, was measured by using the Malvern Zetasizer (Nano ZS 90, Malvern Ltd., Malvern, UK).
Determination of Entrapment Efficiency
For determination of entrapment efficiency QUE-NLCs dispersion was centrifuge at 45,000 rpm for 20 mins; 1.0 mL of the supernatant collected after centrifugation was diluted with 3.0 mL of methanol and then make up volume up to 10 ml in 10ml volumetric flask and measured spectrophotometrically at 256 nm using UV-Visible spectrophotometer (UV 1700, Shimadzu, Japan). Drug loading (DL) refers to the percentage of drug incorporated into the lipid nanoparticles relative to the total weight of the lipoidal phase (i.e., lipid+drug). For this, QUE from lyophilised powder was extracted by triturating 10 mg powder with methanol in mortar pestle and diluted up to 10 ml in volumetric flask. QUE content in the methanolic extract was analysed spectrophotometrically (UV 1700, Shimadzu, Japan) at 256 nm, against the standard methanolic solution of QUE [12].
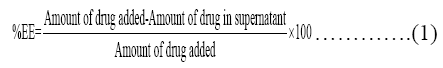
Scanning Electron Microscopy
The surface morphology of freeze-dried optimized formulations was studied using a scanning electron microscopy (JSM 6390®, JEOL DATUM Ltd., Japan). Lyophilized powder of QUE- NLC was dusted onto double-sided tape on an aluminium stub and coated with gold using a cold sputter coater in SEM chamber to a thickness of 400°A, and then photomicrographs were captured by operating at an accelerating voltage of 15 kV electron beam.
X-ray Diffraction Studies
X-ray diffraction patterns of QUE, freeze-dried blank NLCs and QUE-loaded NLCs formulation were obtained using X-ray diffractometer (Bruker Axs, D8 Advance; Germany) in which Cu-Kα line used as a source of radiation by operating at the voltage 40 kV and the current applied was 30 mA. All samples were measured in the 2θ angle range between 10° and 60° with a scanning rate of 3°/min and a step size of 0.02° [13].
In Vitro Drug Release Study
In vitro drug release of the NLC was performed using Franz diffusion cell with dialysis nasal fluid (SNF, 25 ml) for 24 h prior to experiment. In diffusion cell the donor compartment contained the freeze-dried sample while the receptor compartment was filled with simulated nasal fluid (SNF). The donor chamber was placed in such a way that it just touched the diffusion medium in the receptor chamber. The temperature was maintained at 37 ± 0.5°C with the help of a circulating water bath. Samples were periodically withdrawn from the receptor compartment, replaced with the same amount of fresh buffer solution, and assayed using UV spectrophotometer at 650 nm [14].
Ex Vivo Drug Permeation Studies
Fresh nasal tissues were carefully removed from the nasal cavity of sheep obtained from the local slaughterhouse. Tissue samples were inserted in Franz diffusion cells displaying a permeation area of 0.785 cm2. Simulated nasal fluid (25 ml) at 37°C was added to the acceptor chamber. The temperature within the chamber was maintained at 37°C. After a pre-incubation time of 20 minutes lyophilized powder equivalent to 50 mg of QUE was dispersed in 3 ml of SNF and placed in the donor chamber. At predetermined time points, 3 ml samples were withdrawn from the acceptor compartment, replacing the sampled volume with SNF pH 6.4 after each sampling, for a period of 6 hours. The samples withdrawn were filtered and used for analysis. Blank samples (without drug) were run simultaneously throughout the experiment to check for any interference. The amount of permeated drug was determined using a UV-Visible spectrophotometer at 256 nm [15].
The permeation coefficient was calculated by using the formula:-
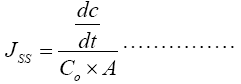 (2)
(2)
Where, JSS is Permeability coefficient, Co is Initial concentration in donar compartment, A is area of mucosal surface, dc/dt is rate of permeability.
Histopathological Examination
Histopathological studies were carried out using isolated sheep nasal mucosa. Freshly isolated sheep nasal mucosa was sectioned in four pieces. Each piece was treated with NP suspension containing QUE at 2 mg ml, drug solution (2 mg ml) in PBS pH 6.4, (as negative control) and isopropyl alcohol (nasal mucociliary toxicity agent used as a positive control), respectively. After treatment for 2 hrs, all the samples were washed properly with distilled water, sectioned and stained with haematoxylin and eosin. The mucosa was then dissected out, and the mucocillia was examined on an optical microscope by a pathologist blinded to the study [16].
In Vitro Cell Growth Assay of QUE NLCs in U373MG Cell-line
Cell growth assay of QUE-NLCs was carried out using astrocytoma-glioblastoma cell line (U373MG). The cells were seeded into 96-well plates at a density of 1.0 × 104 cells per well. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL penicillin, 200 μg/mL streptomycin, 2 mM L-glutamine; and culture was maintained in a humidified atmosphere with 5% CO2. The cells were divided into two treatment groups, including: (a) QUE suspension and (b)Freeze dried QUE -NLCs suspension and % inhibition was determined by MTT[3-(4, 5- dimethylthiazol- 2-yl)-2, 5-diphenyl tetrazolium bromide] assay based on mitochondrial reduction of yellow MTT tetrazolium dye to highly coloured blue formazan product. The plates were incubated with compounds with series of concentrations tested for 48 h at 37 ± 0.5°C in RPMI/DMEM/ MEM with 10% FBS medium. Then the above media was replaced with 90 μL of fresh serum free media and 10 μL of MTT reagent (5 mg/ mL) and plates were incubated at 37 ± 0.5°C for 4 h, there after the above media was replaced with 200 μL of methanol and incubated at 37 ± 0.5°C for 10 min. The absorbance at 256 nm was measured on a spectrophotometer (spectra max, Molecular devices) [17].
Brain Distribution Studies
Male wistar rats weighing 250-270 g were selected for the bio distribution studies which were divided into two groups, one for formulation and other for drug suspension respectively. The rats were anaesthetized with an intraperitoneal injection of pentobarbital (40 mg/kg) and kept on a heating pad to maintain the body temperature. To Group I, 100 μl of dispersion containing lyophilised powder equivalent to 10 mg QUE were instilled into the nostrils with the help of micropipette attached with LDPE tubing, having 0.1 mm internal diameter at the delivery site. The rats were held from the back in slanted position during intranasal administration. For Group II the 100 μl quercetin suspensions (equivalent to 10 mg/ml) were instilled in to the nostrils in a same manner. The rats were sacrificed humanely, at different time intervals and skull was cut open and the brain was carefully excised. Each brain tissue was quickly rinsed with saline and blotted up with filter paper to get rid of bloodtaint and macroscopic blood vessels as much as possible and weighed. After weighing, the brain tissue samples were homogenized with 1 volume of saline in a tissue homogenizer (Teflon homogenizer). At each time point, 3 rats were taken for measurements. All brain homogenates were stored for up to 48 h in a deep freezer (-70°C) until HPLC analysis.
Processing of samples: Brain was isolated and placed into pre-weighed scintillation vials. The organ samples were made homogenate and QUE in tissue homogenate was subsequently extracted by methanol, 10 mol/l HCl and analysed by HPLC method. During the analysis, each 400 μl organ sample was mixed with1.0 ml methanol containing 400 μl 10 mol/lHCl. After mixing well, the sample was incubated at 90°C for 5 h and 1.0 ml ethyl acetate was added. After centrifugation at 5000 rpm for 10 mins, supernatant of organ sample was dried and reconstituted in 500 μl methanol for HPLC analysis [18].
Chromatographic conditions: QUE concentrations in the brain homogenate were determined using HPLC system reported. The drug concentration in plasma was analysed by a high performance liquid chromatography (HPLC) method using Rheodine type manual injector. The HPLC system (Agilent 1200 Series) consisted of C18 column (Eclipsed XDB 5 μm, 4.6 mm × 150 mm, Singapore), Ezchrome Elite Software, quaternary pump, Model G1354 A and Ultraviolet variable wavelength Diode Array detector, Model G1315D. The detection wavelength was 256 nm. The mobile phase was composed of 30% methanol/20% acetonitrile/50% (5%) phosphoric acid from 0 to 15 min. Elution was performed isocraticallyat a flow rate of 1.0 mL/min.
Data analysis: Pharmacokinetic parameters were analyzed by noncompartmental analysis. The maximum observed plasma concentration Cmax and the time to reach Cmax (tmax) were determined directly from the data. Trapezoidal method was used to calculate the concentrationtime curve from time 0-24 h. (AUC0-24). The Kinetica 5® (Thermo Fisher Scientific Demo version, Thermo Fisher Scientific, Waltham, MA) software was employed for the study.
Statistical analysis: Results are expressed as the mean ± SD (Standard deviation) of at least three experiments. Statistical significance was assessed using the student’s t-test for multiple comparisons with p<0.05 as the minimal level of significance.
Results and Discussion
QUE-loaded NLCs were prepared using hot high pressure homogenization technique. We have screened many lipid materials including GMS, Compritol 888 ATO, stearic acid, palmitic acid, castor oil, oleic acid, capmul GMO Lipoid, Polaxomer 188, and Tween 80 were also screened as surfactants. On basis of solubility study in these lipids GMS was selected as solid lipid and capmul GMO as liquid lipid. Polaxomer 188 and soya lecithin were selected as surfactant and stabilizer, respectively. For the stability of dispersions balance of emulsifiers is required at the oil-water interface. Various methods were used for the preparation of NLC such as hot and cold homogenization, solvent diffusion, and micro emulsion. We have selected hot high pressure homogenization technique for preparation of QUE-NLC as it is simple and quick at laboratory scale; also there is no use of organic solvents in the development of formulation. The homogenizer pressure was optimized to 600 bars for ten cycles at 75 ± 0.5°C. As formulations were produced at 75°C to ensure that the lipids remain in the liquid state during the production process.
Particle Size and Polydispersity Index
The size of the QUE-NLC was about 118.2 nm with poly dispersity index of 0.220. As the particle size of the NLCs dispersion is a crucial factor because it determines the rate and extent of drug release as well as drug absorption. It has been reported that the smaller particle size may lead to more rapid absorption and improve the bioavailability. QUE NLC has zeta potential of -20.1 mV. It is currently admitted that higher ZP values, either positively or negatively charged, mean that dispersion will have greater long-term stability.
Determination of Entrapment Efficiency
The entrapment efficiency of quarcetin in nanostructure lipid carriers was found to be 88.74%. In case of QUE-NLCs, as the concentration of GMS increases EE was also increased. But, as the amount of Capmul GMO was goes above 35%, EE was decreased even though concentration of GMS was increased. This attributed to lipid precipitation mechanism that occurs during particle development.
Scanning Electron Microscopy
Morphological study of QUE-NLC was done by taking SEM photograph (Figure 1). It was revealed that they were needle like in shape. Studies also verified that the crystalinity of QUE decreased as it was encapsulated by lipids.
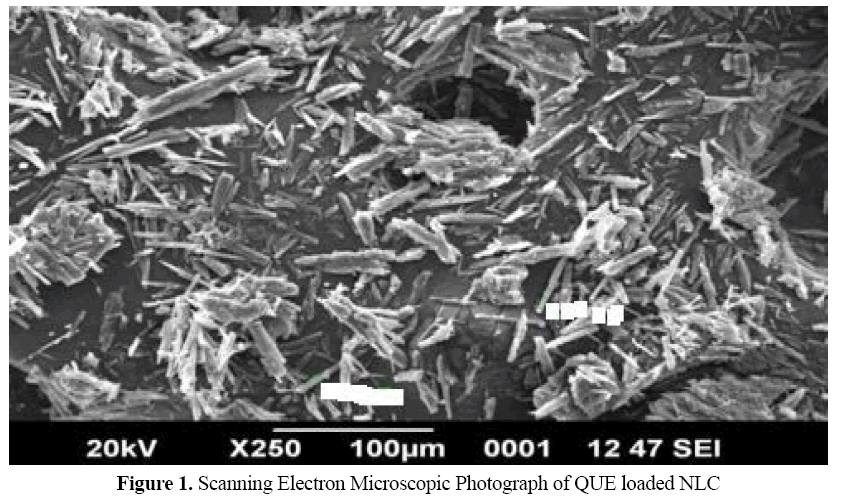
Figure 1: Scanning Electron Microscopic Photograph of QUE loaded NLC
X-ray Diffraction Studies
The X-ray diffraction pattern of the quercetin shows the presence of high intensity peak which suggest that high crystalline form of the drug (Figure 2). The lyophilized QUE-NLC also showed the similar peaks but with slightly different peak intensities. It suggests that decrease in crystalinity in QUE-NLC formulation.
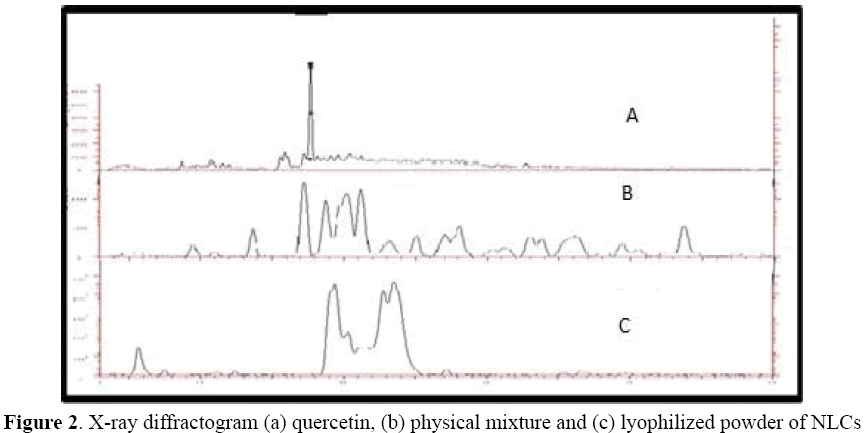
Figure 2: X-ray diffractogram (a) quercetin, (b) physical mixture and (c) lyophilized powder of NLCs
In Vitro Drug Release
On comparison of release of QUE from drug loaded NLC and pure drug suspension (PDS) through the dialysis membrane in SNF (pH 6.4), NLCs formulations showed sustained release at the constant rate for 6 hrs (Figure 3).
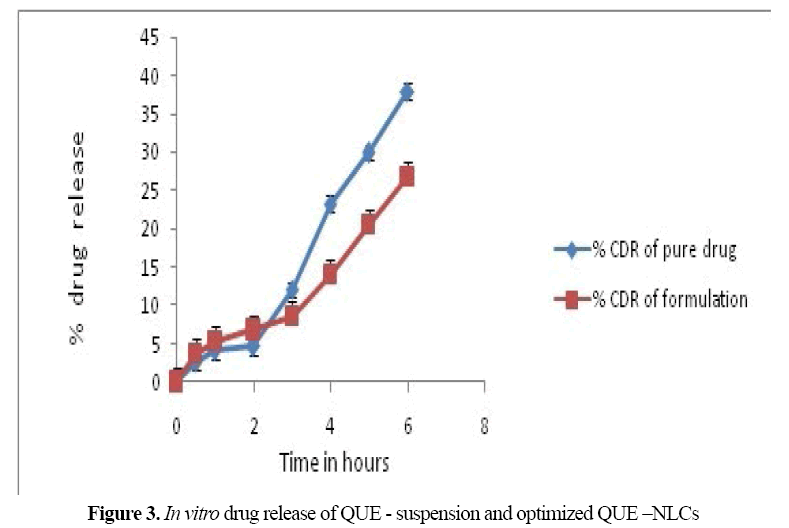
Figure 3: In vitro drug release of QUE - suspension and optimized QUE –NLCs
Ex Vivo Permeation Studies
Ex vivo nasal permeability data reveals that quercetin diffused at faster rate and the total percentage diffusion was much higher from the nanostructure lipid carrier system than pure drug suspension. After 6 hours of diffusion 76.71 ± 1.97% of QUE was diffused from QUE loaded NLC and 26.73 ± 3.60% form pure drug suspension. Permeability coefficient was found to be 0.38 cm/min for NLC formulation.
Histopathological Studies
Histological studies show negative control mucosa (normal nasal mucosa) and positive control (mucosa stained with haematoxylineosin) and the effect of formulation on sheep nasal mucosa, 2 hrs after applying the formulations. No change in mucosal structure was seen when treated with QUE loaded NLC as compared to the positive control. The section of mucosa treated with formulation QUE-NLC showed no changes in nasal epithelium (Figure 4). There was no sign of remarkable destructive effect of formulations on the treated nasal mucosal damage like inflammation or necrosis.
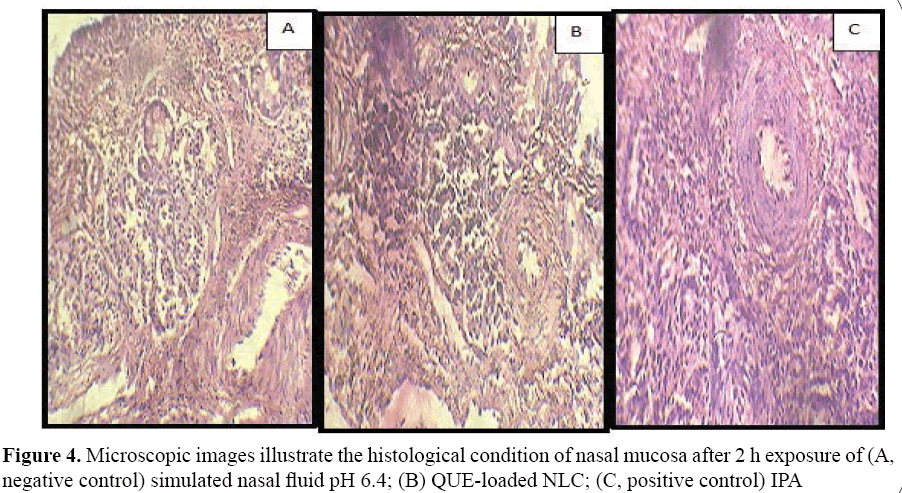
Figure 4: Microscopic images illustrate the histological condition of nasal mucosa after 2 h exposure of (A, negative control) simulated nasal fluid pH 6.4; (B) QUE-loaded NLC; (C, positive control) IPA
In Vitro Cell Growth Assay of QUENLCs in U373MG Cell-line
In vitro cell growth assay of freeze dried QUE - NLCs suspension was examined and compared with standard i.e., adrenomycin by cell viability testing. Results shows graph of % inhibition of astrocytoma-glioblastoma cell line (U373MG) incubated with the QUE-NLCs and adrenomycin vs. various drug concentrations (Figure 5). From results, we can see that at the same concentration, QUE - NLCs produced cell inhibition on the cells when the drug concentration is 40 μg/ ml. The quercetin NLCs are able to cause cell inhibition. To explain the cell growth inhibition effect of NLCs, it was hypothesized that NLCs absorbed to cell surface and released QUE close to the membrane, leading to a high local drug concentration gradient or was brought in the cells and then released from the NLCs [18,19].
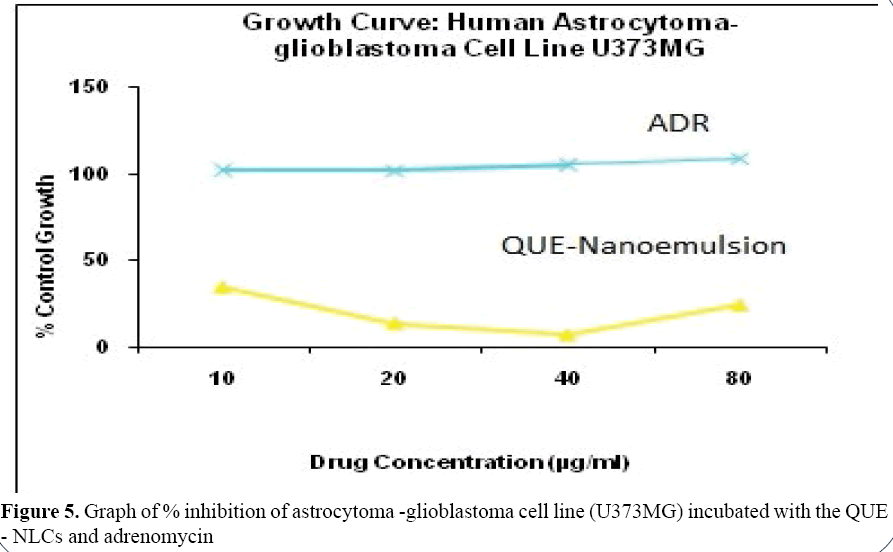
Figure 5: Graph of % inhibition of astrocytoma -glioblastoma cell line (U373MG) incubated with the QUE - NLCs and adrenomycin
Brain Distribution Study
The profile of quercetin concentration in brain of drug loaded NLC (QUE-NLC) and quercetin suspension vs time depicts the results of bio distribution studies following nasal administration. Quercetin concentration in the brain was found to be higher for QUE-NLC (82.67 ± 19.12 μg/g) and plain quercetin suspension (18.53 ± 9.54 μg/g) after the initial 30 mins on nasal delivery. The pharmacokinetic profiles displayed that Cmax concentration achieved with in 60 min in brain. As time progressed, the enhancement of brain concentration was observed and after 60min, higher brain uptake (93.637 ± 19.88 μg/g) of quercetin as compared to plain quercetin suspension (42.26 ± 99.04 μg/g). The Cmax was 93.63 ± 19.88 μg/g at tmax of 60 min and 42.26 ± 99.04 μg/g at tmax of 90 min respectively for NLCs and PDs after IN administration. The elimination half lives (t1/2) of both formulations are comparable (Table 1) demonstrating no effect formulation type on rate of elimination of drug. A statistically significant difference (P<0.05) between the two formulations was found from the student t-test analysis. Improved quercetin delivery to CNS after intranasal administration of QUE-NLC (AUC0-∞- 55272.709 ± 100.99 μg/ml×min) as compared to quarcetin suspension (AUC0-∞ 23882.62 ± 20 μg/ml×min) was attributed to rapid diffusion of QUE-NLC in nasal cavity that provides targeted delivery to brain. Moreover small size of NLCs allows transcellular as well as paracelluar transport via olfactory neurons in the olfactory membrane. On the basis the results of brain distribution studies it was proved that on intranasal delivery quercetin could be transported directly to the CNS, resulting increased drug concentration in the brain and also enhancing the CNS availability of quercetin (i.e., 231.43 ± 4.53) (Table 1). Studies show that direct delivery of the drug molecules from nose to brain is of vital importance for the targeted delivery to CNS bypassing BBB [20,21].
Table 1. Pharmacokinetic parameters following intranasal administration of QUE loaded NLC and plain drug suspension
| Pharmacokinetic Parameters |
QUE-NLCs |
QUE suspension |
| Cmax ± SD (µg/ml) |
93.63 ± 19.88 |
42.26 ± 9.90 |
| Tmax ± SD (min) |
60 ± 2.31 |
90 ± 5.773 |
| T1/2 (min) |
366.37 ± 5.06 |
358.85 ± 5.47 |
| AUC0-t (µg/ml × min) |
32220.66 ± 66.14 |
13122.28 ± 65.73 |
| AUC0-∞ (µg/ml × min) |
55272.70 ± 100.99 |
23882. 62 ± 20 |
| Fr (%) n=3 |
231.43 ± 4.53 |
- |
Conclusion
QUE-NLC with narrow size and high drug entrapment efficiency were formulated. The drug releases of QUE-NLC exhibit sustain release behaviour. Bio distribution studies reveals contribution of NLC in drug targeting to brain with improved availability. It can be concluded that the QUE-NLC might be a promising nano carrier for effective targeting to tumour.
Acknowledgement
One of the author (Hitendra S Mahajan) thankful to University Grant Commission for providing financial assistance under Research Award Scheme (F. 301/2014(SAII)/ RA201416OBMAH5821).
Conflict of Interest
The authors report no declarations of interest.
References
- Abdelwahed W, Degobert D, Stainmesse S, et al. Freeze drying of nanoparticles: Formulation,process and storage considerations.Adv Drug Deliv Rev. 2006; 58:1688-713.
- Aditya NP, MacedoAS, Doktorovova S, et al. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT-Food Sci Tech. 2014;5:1-7.
- Bose S, Michniak-Kohn B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur J Pharm Sci. 2012;48:442-52.
- Charlton ST, Davis SS, Illum L. Evaluation of bioadhesive polymers as delivery systems for nose to brain delivery: In vitrocharacterisation studies. J Control Release. 2007;118:225-34.
- Cirillo G, Vittorio O, Hampel S, et al. Quercetin nanocomposite as novel anticancer therapeutic: Improved efficiency and reduced toxicity. Eur J Pharm Sci. 2013;49:359-65.
- Das S, Chaudhary A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharm Sci Tech. 2011;12(1):62-77.
- Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2010;63:342-51.
- Gang W, JueWJ, Ping ZL, et al. Liposomal quercetin: evaluating drug delivery in vitro and biodistribution in vivo. Expert Opin Drug Delivery. 2012;9(6):599-613.
- Hertzog DI,Tica OS. Molecular mechanisms underlying the anticancerous action of flavonoids. Curr Health Sci J. 2012;38(4):145-149.
- Illum L.Nasal drug delivery-possibilities, problems and solutions. J Control Release. 2003;8:187-198.
- Kabita KK, Samuel R,Karunakar H, et al. A comprehensive review on brain tumor. International J Pharm Chem Biol Sci. 2013;3(4):1165-1171.
- Kalam MA, Sultana Y,Ali A, et al. Preparation, characterization, and evaluation of gatifloxacin loaded solid lipid nanoparticles as colloidal ocular drug delivery system. J Drug Target. 2010;18(3):191-204.
- Khonkarn R, Mankhetkorn S, Hennink WE, et al. PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Euro J Pharm Biopharma. 2011;79:268-275.
- Li C.Poly (L-glutamic acid)-anticancer drug conjugates. Adv Drug Deliv Rev. 2002;54:695-713.
- Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597-611.
- Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135-146.
- Neethu CS, Kavitha VS,KrishnakumarK, et al. Quercetinnanocrystal formulation: In vitro anti-tumour activity against dalton lymphoma cells. J Drug Discov Therap. 2015;3:9-17.
- Patil GB, Patil ND, Deshmukh PK, et al. Nanostructured lipid carriers as potential vehicle for Carvediloldelivery: Artificial Cells. Nanomed Biotechnol. 2014;19:1-8.
- Sahiner N.One step poly(quercetin) particle preparation as bio colloid and its characterization, colloids and surfaces. Physicochem Eng Asp. 2014;173-180.
- Seju U, Kumar A, Sawant KK. Development and evaluation of Olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro an in vivo studies. Acta Biomaterialia. 2011;7:4169-4176.
- Tas C, Ozkan CK, Savaser A, et al. Nasal absorption of metoclopramide from different Carbopol 981 based formulations: In vitro, Ex vivo and in vivo evaluation. Euro J Pharm Biopharma. 2006;64:246-254.

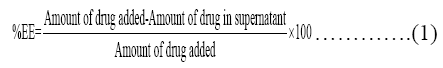
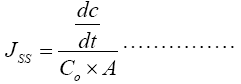 (2)
(2)