Case Report - (2018) Volume 5, Issue 1
Cvetka Graši? Kuhar1*, Klara Geršaka2, Živojin Stevanovi?1 and Barbara Gazi?c3
1Institute of Oncology Ljubljana, Department of Medical Oncology, Zaloška 2, SI-1000 Ljubljana, Slovenia
2General Hospital Izola, Polje 40, SI-6310 Izola, Slovenia
3Institute of Oncology Ljubljana, Department of Pathology, Zaloška 2, SI-1000 Ljubljana, Slovenia
Corresponding Author:
Cvetka Graši? Kuhar
Institute of Oncology Ljubljana, Department of Medical Oncology, Zaloška 2
SI-1000 Ljubljana, Slovenia
Tel: +38615879282
Fax: +38615879303
E-mail: cgrasic@onko-i.si
Received date: February 19, 2018; Accepted date: March 03, 2018; Published date: March 07, 2018
Citation: Kuhar CG, Geršaka K, Stevanovi? Z, Gazi?c B (2018) Leukocytoclastic Vasculitis Associated with Exemestane/Everolimus Therapy in a Previously Irradiated Skin: Case Report. Br J Res Vol. 5 No. 1:37. doi: 10.21767/2394-3718.100037
Copyright: © 2018 Kuhar CG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Leukocytoclastic vasculitis may be secondary to medications, underlying infection, collagen-vascular disorders, or malignancy. We report the second case of leukocytoclastic vasculitis related to exemestane/ everolimus therapy in breast cancer and present our review of literature.
Case presentation: A 68-year-old Caucasian woman, treated with exemestane and everolimus for metastatic breast cancer presented with skin necrosis and allodynia in the right mammary region. A biopsy disclosed leukocytoclastic vasculitis. The patient received radiation therapy to the right breast due to exulcerated breast cancer (20 × 2.5 Gy, total dose 50 Gy) two and a half years ago. Skin necrosis appeared after five days of treatment with everolimus and 22 days of exemestane. The treatment with exemestane was continued, but everolimus was discontinued. Allodynia disappeared in two weeks after everolimus discontinuation. The skin healed very slowly and an eschar persisted for several months. The prompt resolution of allodynia, evident healing of skin necrosis after everolimus discontinuation, and high score in the Naranjo Adverse Drug Reaction Probability Scale strongly suggest the causative role of everolimus in leukocytoclastic vasculitis.
A review of literature revealed only one case of leukocytoclastic vasculitis caused by everolimus and one case of radiation recall dermatitis caused by exemestane/ everolimus. Even though it is a serious adverse effect, it is usually reversible by drug discontinuation and symptomatic treatment only.
Conclusion: Everolimus-related skin recall presented as leukocytoclastic vasculitis is a very rare adverse drug reaction.
Keywords
Leukocytoclastic vasculitis; Everolimus; Exemestane; Radiation recall; Breast cancer
Introduction
Everolimus, a selective mammalian target of rapamycin (mTOR) inhibitor, in combination with exemestane, has been approved for the treatment of advanced hormone receptor positive, HER2/neu negative breast cancer [1].
We report the case of a patient who developed cutaneous leukocytoclastic vasculitis (LCV) after 45 and 28 days of treatment with exemestane and everolimus, respectively. We consider this case relevant to present to other oncologists in order to alert them of this potential adverse effect on a previously irradiated skin that can only be treated with drug discontinuation.
Case Report
A 68-year-old Caucasian woman without other known diseases or previous medical history of hepatitis, HIV infection, or autoimmune disease, without regular medical therapy (except for three years of contraceptive pill usage in the 1970s), not using any home remedies, without any known allergies, mother to three biological children, was diagnosed with exulcerated cancer of the right breast in December 2014, following four years of breast lump neglect. At presentation, the breast tumor invaded the sternum, upper anterior mediastinum, right supraclavicular region, and right axilla, causing lymphedema of the right arm. The bone scan was suspicious for bone metastases. The tumor was hormone receptor positive and the HER2 test failed many times. The patient was treated with systemic therapy (letrozole) and locally with radiation therapy to the right breast, axilla, and mediastinum (20 × 2.5 Gy, total dose 50 Gy). A partial remission was achieved but, in December 2015, the cancer progressed in the right supraclavicular region. Systemic treatment was changed to tamoxifen. Additionally, the patient received radiation to the right supraclavicular region (28 × 2 Gy, total dose 56 Gy). In April 2017, the cancer progressed in the patient’s bones and the right supraclavicular region. The therapy was changed to exemestane/everolimus and zoledronic acid. To avoid the concomitant use of everolimus during radiation therapy to the spine (L2-S2, 5 × 4 Gy, total dose 20 Gy), the beginning of the everolimus therapy was postponed to seven days after the conclusion of radiation treatment. Five days into everolimus therapy and 22 days into exemestane therapy, a painful wound measuring 7 × 5.5 cm with an abundant serous secretion arose in the previously irradiated right mammary region. The patient continued with therapy until the planned visit on the 28th day of everolimus (Figure 1A) therapy. She was not exposed to any other drugs during the same time period. Upon physical examination, the patient had allodynia (an intractable pain on mild touch but no pain otherwise). The medical oncologist decided to discontinue the administration of everolimus, but not exemestane and zoledronic acid because of their prompt effect on tumor marker CA 15-3. A wound biopsy was performed, disclosing leukocytoclastic vasculitis with a deep and superficial dermal necrosis. A low power view showed a population of predominantly neutrophils in a perivascular and interstitial pattern in addition to those undergoing extravasation from the vessels. Fibrinoid necrosis of the vessels with fibrin extravasation was also present (Figure 1B).
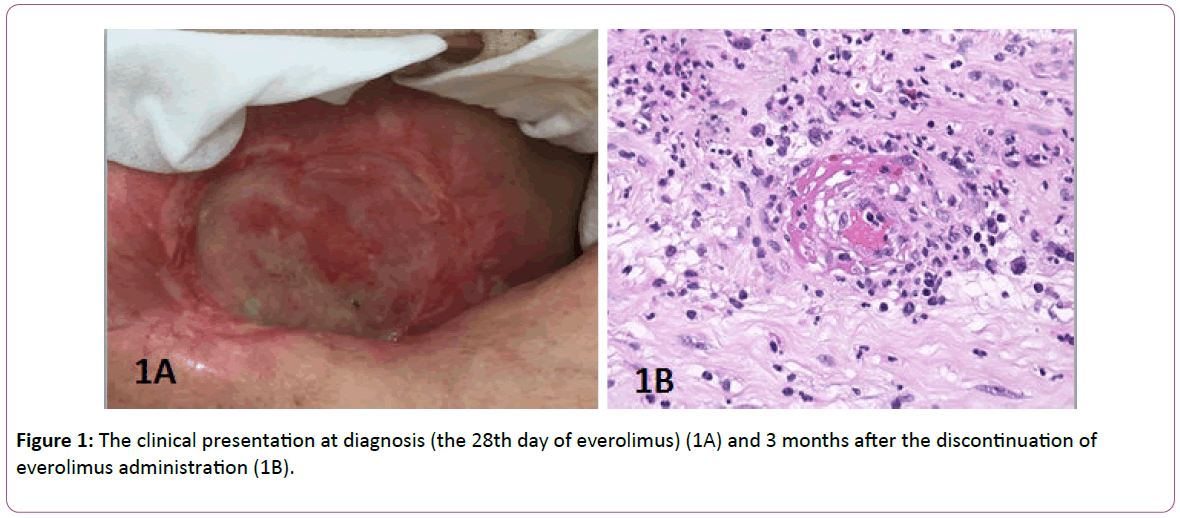
Figure 1: The clinical presentation at diagnosis (the 28th day of everolimus) (1A) and 3 months after the discontinuation of everolimus administration (1B).
No tumor cells, bacteria, or fungi were found in the biopsy specimen. Direct immunofluorescence (DIF) was not performed because only formalin-fixed tissue was available. DIF studies are possible only on the frozen tissue.
A wound smear revealed a Staphylococcus aureus superinfection, which was treated according to antibiotic susceptibility test with amoxicillin/clavulanic acid. Allodynia disappeared completely in two weeks, but the wound healed more slowly and incompletely (Figure 2).
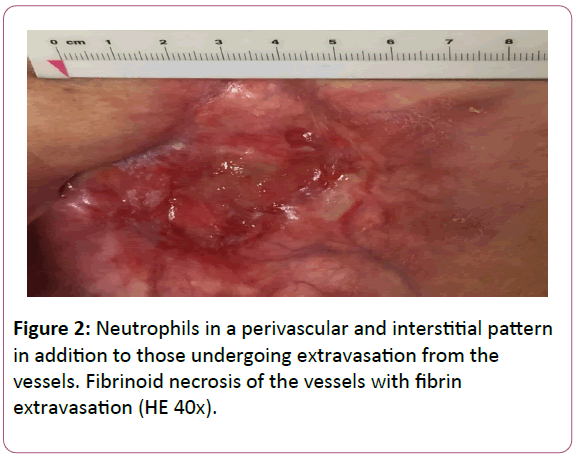
Figure 2: Neutrophils in a perivascular and interstitial pattern in addition to those undergoing extravasation from the vessels. Fibrinoid necrosis of the vessels with fibrin extravasation (HE 40x).
Additional blood tests were performed: urea, creatinine, antinuclear antibody (ANA), antibodies to extractable nuclear antigens (ENA), antineutrophyl cytoplasmic antibody test (ANCA) and rheumatoid factor. All of the tests had normal or negative results. No steroids or immunosuppressive therapy were administered due to the absence of systemic manifestations of vasculitis.
Exemestane monotherapy was not effective in our patient. After 5 months CT scan showed disease progression with lung metastases and therapy was changed to capecitabine. We evaluated the likelihood of everolimus having caused LCV by using the Naranjo Adverse Drug Reaction Probability Scale [2]. The probability score was 7, which means that LCV was 'probably' related to everolimus (Table 1).
| Yes | No | Do not know | Score in this case | ||
|---|---|---|---|---|---|
| 1 | Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | +1 |
| 2 | Did the adverse event occur after the suspected drug was administered? | +2 | -1 | 0 | +2 |
| 3 | Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | +1 |
| 4 | Did the adverse reaction reappear when the drug was re-administered? | -1 | +2 | 0 | 0 |
| 5 | Are there alternative causes (other than the drug) that could have, on their own, caused the reaction? | -1 | +2 | 0 | +2 |
| 6 | Did the reaction reappear when a placebo was given? | -1 | +1 | 0 | 0 |
| 7 | Was the drug detected in the blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| 8 | Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9 | Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 0 |
| 10 | Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | +1 |
| Total score | +7 | ||||
Total score: >8: definite, 5-8: probable, 1-4: possible, 0: doubtful.
Table 1: The scoring of adverse drug reaction of everolimus according to the Naranjo Adverse Drug Reaction Probability Scale.
As the clinical presentation was severe, we did not expose the patient to further treatment with everolimus that would have given a definite diagnosis. The patient signed an informed consent for publishing her case in a medical journal. The patient’s rights to privacy were respected.
Discussion
When taking into consideration the medical history and clinical presentation, the cause of LCV in our patient was probably everolimus. The time between the first exposure to everolimus and the clinical presentation was 5 days, even though the patient delayed their visit as pre-planned (on the 28th day). The first case of LCV caused by everolimus 5 mg was reported by Yee et al. in 2005, namely in a patient with myelodysplastic syndrome. That patient presented with no systemic manifestation of vasculitis and did not receive any systemic immunosuppressive therapy, but skin grafting was performed after 25 weeks [3]. In our case, as well, no systemic manifestations were detected and the patient was not treated with systemic steroids. Only local measures were taken. After 8 month of everolimus discontinuation, a small cutaneous erosion still persisted. Our case is special as vasculitis manifested itself in a previously irradiated skin. Similarly, Ioannidis et al. reported a case of radiation recall vasculitis of CTCAE grade 2 after only 3 days of treatment with everolimus and exemestane in a mammary region irradiated 10 years ago [4]. They did not perform a biopsy. Recall dermatitis disappeared after 2 weeks of discontinuation with everolimus and exemestane and therapy with steroids. On rechallenge of exemestane and lower dose of everolimus (5 mg), recall dermatitis did not recur [4]. Bourgier et al. reported ‘in-radiation-field’ gastric mucocal inflammation and ulcers in a breast cancer patient, treated with paclitaxel, trastuzumab and everolimus. Additionally, two cases of mucosal reaction within pre-irradiated tissue with another mTOR inhibitor temsirolimus was presented [5]. The unanswered question is why our patient manifested with LCV only in the mammary-axillary region but not in the supraclavicular region, despite both regions having been irradiated in the past. The mammary-axillary region was irradiated 2.5 years before everolimus (2.5 Gy × 20), and the right supraclavicular region 1.5 years before everolimus (2 Gy × 25). In the supraclavicular region, there were no clinical signs suspicious of LCV, nor were there any signs of recall dermatitis. The only difference between both regions was the dose of radiation per fraction: maybe the higher dose predisposed to LCV. Whether it was recall dermatitis which led to LCV remains an unsolved question. Lee et al. presented a patient with a localized Sweet syndrome three years after chemoradiation for cervical cancer in the previously irradiated skin [6]. Similar to our case, a skin biopsy showed dense dermal infiltrates composed mostly of neutrophils but without evidence of true vasculitis.
According to the Naranjo Adverse Drug Reaction Probability Scale, it is probable that cutaneous LCV in our case was caused by everolimus. Negative blood tests excluded other forms of vasculitis (ANCA vasculitis and vasculitis associated with systemic diseases). Direct immunofluorescence was not performed, because only formalin-fixed tissue was available. It could be performed on frozen sample only. It might provide some additional information, but they would not be decisive in the interpretation of the case anyway.
Cutaneous LCV is very rarely caused by breast cancer medications [7]. Santoro et al. presented skin adverse reactions of aromatase inhibitors. Among them were anastrazole, letrozole, and exemestane, and each of them was related to vasculitis in one case [7]. In the literature, there is no report of LCV caused by zoledronic acid. Exemestane and zoledronic acid were both continued in our patient and LCV did not recur. The probability that exemestane or zoledronic acid caused LCV is, according to the Naranjo probability scale, in the range of possible only (score 1-4) [2]. LCV usually has a favorable course and rarely requires an aggressive therapy. Corticosteroids are indicated when there are signs of incipient skin necrosis [8].
Conclusion
Our research reports the second case of LCV after treatment with everolimus and the second case of adverse reaction in a previously irradiated mammary region. Our case is the first biopsy proven report of cutaneous LCV in the previously irradiated skin. Medical oncologists should be aware of this potentially serious side effect of everolimus.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of Interest
CGK has received honoraria from Novartis. Other authors declared no financial disclosure.
Disclosure
All authors participated in the preparation of the article and have approved the final manuscript.