Group 15 polymers; Pancreatic cancer; Histamine; Interfacial polymerization; MALDI MS; Breast cancer
Introduction
We have been synthesizing metal-containing polymers for a number of reasons. Most recently our focus has been on synthesis of polymers to combat unwanted pathogens and infectious agents. Here the focus is cancer. By rationalizing the synthesis of metal containing moieties of known biological activities like Lewis base showing the synergetic affect. The Group 15 elements which are known for the biological activities for instance [1-4], the topics of the group 15 elements containing polymers has recently been reviewed which are synthesized from simple reactions of Organometallic dihalide or dinitrate with Lewis base, ex: Diamines and Diols [4-14]. Hereafter stated to emphasize Group-15 containg polymers exhibiting inhibitions of pancreatic, breast, prostate, lung and other cancer cell lines [15-19].
Histamine (2-aminoethylimidazole; Figure 1) is involved in complex biological processes where it interacts with specific receptors on cell membranes [20-24]. Histamine is believed involved with a variety of human pathologies including asthma, allergies, acid-peptic disorders, and duodenal ulcers. It is an amino acid that is a neurotransmitter or neuromodulator largely synthesized in mast and white blood cells. It increases the permeability of capillaries in white blood cells increasing their ability to act as a defense to certain pathogens. Hence it fits our criteria of exhibiting some biological activity on its own.
Figure 1: Space filled model of triphenylantimony dichloride and histamine.
A number of reports describe incorporating the histamine moiety into polymers. Most of these involved two approaches. The first approach is the coordination of histamine or histaminecontaining molecules with various metal ions. For instance Modder, Koten and others reported on the formation of a number of coordination polymers formed by binding of peptidebased polydentate ligands through a self-assembly process containing the histamine moiety using silver and copper ions [25]. There were a variety of structures formed depending on reaction conditions. Jeong, Ha and coworkers described the formation of Langmuir-Blodgett films from the coordination of potassium, iron, and other ions onto the polymer poly(N-(2-(4- imidazoly)ethyl)maleimide-alt-1-octadecene) that has the histamine moiety “hanging” from the main polymer chain [26]. The films have decent stability withstanding a surface pressure of 40 to 50 mN/m. Self-assembly ladder-like coordination polymers formed though reaction with copper+2 were reported by Min and Suh [27]. The products show ferromagnetic interactions dependent on the particular part of the chain measured.
The second approach concerned incorporating the histamine moiety as a side-unit on an already existing polymer. Much of this work was aimed at developing molecules of potential biological importance. Anderson, Lynn, and Langer formed a number of cationic polymers for gene delivery including those containing the histamine moiety using a semi-automated synthetic approach [28]. Combinatorial, automated highthroughput synthesis was employed to synthesize and screen the products. Nelson, Lavigne and coworkers synthesized a number of polymers from the reaction of diamine-containing reactants, including histamine, with reaction through the acid group on carboxylic acid-substituted poly(thiophene) [29]. Wong and Putnam also incorporated the histamine moiety on the sidechain of a polymer, in this case polymethacryhloxysuccinimide [30]. The effort studied the delivery of histamine and other sidechain units. Chen and coworkers studied the effect of proton transport by supramolecular nanochannels in comb polymers including histamine units [31].
This paper describes the synthesis of Group 15-containing polyamines from reaction between the histamine and triphenylmetal dihalides (Figure 2).
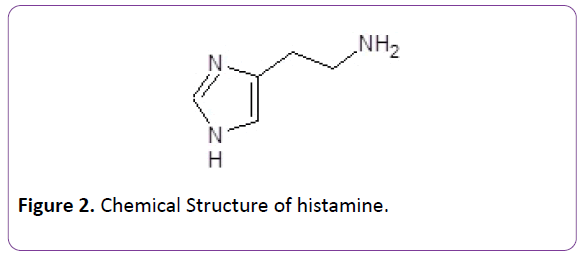
Figure 2: Chemical Structure of histamine.
Experimental
Synthesis
The interfacial polycondensation technique was employed to synthesize the polymers. Initially, histamine (0.00200 mole) and sodium hydroxide (0.0040 mole) dissolved in water (20 mL) were added to a one quart Kimax emulsifying jar. The jar was fitted on top of a Waring Blender (model 1120; no load speed of about 18,000 rpm; reactions were carried out at about 25°C). Stirring (about 18,000 rpm) was begun and a chloroform solution (20 ml) for the triphenyl antimony and triphenyl arsenic and carbon tetrachloride for the triphenyl bismuth solution added (about 3-4 seconds) through a hole in the jar lid using a powder funnel. Blending continued for 30 seconds. Vacuum filtration was used to recover the precipitate which was then washed several times with deionized water and chloroform or carbon tetrachloride to remove unreacted materials and unwanted by-products. The solid was transferred to a glass Petri dish and allowed to dry.
Triphenylarsenic dibromide (3313-89-1) was synthesized [32]. Triphenylbismuch dichloride (594-30-9) was purchased from Aldrich Chemical Co., Milwaukee, WS; triphenylantimony dichloride (594-31-0) dichloride was purchased from Strem Chemical Co., Newburyport, MA. Histamine (51-45-6) was obtained from Sigma-Aldrich, Saint Louis, MO.
Physical characterization
Molecular weight was determined in DMSO solution employing light scattering photometry employing A Brice- Phoenix Universal Light Scattering Photometer Model 4000 was employed to obtain molecular weight on dimethyl sulfoxide, DMSO, solutions containing dissolved polymer. A Thermo Scientific Nicolet iS5 FTIR equipped with an id5 ATR attachment. 1H NMR spectra were obtained in d-6 DMSO employing Varian Inova 400 MHz and Varian 500 MHz spectrometers.
A Voyager-DE STR BioSpectrometer, Applied Biosystems (Foster City, CA) was used to obtain high resolution electron impact positive ion matrix assisted laser desorption ionization time of flight, HR MALDI-TOF, mass spectra. An accelerating voltage of 25,000 was used with spectra obtained over a mass range of 500 to 2500 Da. Typically 200 shots were used for each spectra. The matrix material was alpha-cyano-4- hydroxycinnamic acid with the matrix placed in a small glass vial along with copper spheres with shaking resulting in a fine power that was used as the sample.
Cell testing
The toxicity of the test compound was evaluated for a number of cancer cell lines. Cells were placed into a 96-well culture plate at a density of 20,000 cells per 100 μL of culture medium. Following a 24 hr. incubation period, The test compounds were incubated for 24 hours at concentrations ranging from 0.0032 to 32,000 ng/ml and allowed to incubate at 37°C with 5% CO2 for 72 h. Titer-Blue reagent (Promega Corporation) was added (20 μl/well) and the cells incubated for an additional 2 h. Fluorescence was determined at 530/590 nm and converted to % cell viability versus control cells.
To ensure that toxicity was due to the compounds and not diluents, “mock-treatments” were carried out where the same procedure was carried out except omitting the test compound. This mock-treatment never resulted in an inhibition of greater than 1%.
Results and Discussion
Yield and chain length
Table 1 represents yield and chain length of products formed during the reaction of the histamine and Group 15 triphenylmetallic dihalides.
| Lewis Base |
% Initial Yield |
Additional % Yield/2 Days |
Overall % Yield |
Molecular Weight |
DP |
| Ph3AsBr2 |
20 |
4 |
24 |
2.4 × 106 |
5800 |
| Ph3SbCl2 |
10 |
8 |
18 |
7.4 × 104 |
160 |
| Ph3BiCl2 |
13 |
11 |
24 |
4.5 × 106 |
8200 |
Table 1. Polymer product yield molecular weight and chain length as a function of Lewis Base for the reaction between the various Lewis bases and histamine. The particular reaction concentrations and conditions are given the experimental section.
Product is formed in thirty second and less. Yield is in the low range. For polymerizations employing Group 15 triphenylmetallic halides, additional product precipitates from the reaction system after the initial product precipitates from the reaction solution [6,10-19]. For the histamine polymers, the arsenic and bismuth products have high molecular weights while the antimony has only a moderate chain length. It is difficult to describe synthetic trends because two different organic solvents were employed with chloroform used for the antimony and arsenic as the organic liquid while the bismuth system used carbon tetrachloride as the organic liquid since triphenylbismuth dichloride is soluble in carbon tetrachloride but not in chloroform. Triphenylarsenic dibromide is brown because of the presence of the As-Ph brown color site. The bismuth and antimony products are white because of the absence of a color site in the reactants. The products showed good adhesion to the glass Petri dish.
Infrared spectroscopy
Infrared spectra were taken of the monomers and polymers. Results for the arsenic and antimony polymers appear in Table 2. The band at 2968 (all bands are given in cm-1) characteristic of the ring NH stretch in histamine is absent in the spectra of the polymers. Further, the band at 1596 due to NH2 scissoring found in histamine is absent in the spectra of the polymers. Both of these are consistent with the formation of the M-N linkage. The NH ring stretch should be missing because it is no longer present being replaced by the M-N moiety. The aliphatic NH2 is now a NH-M so scissoring is no longer possible. The presence of a new band about 1250 is consistent with the formation of the M-N linkage. Further, bands characteristic of both the histamine and triphenylmetal moiety are present. Band assignments are consistent with reports for other studies [6,10-19,33-37]. Thus, infrared spectroscopy evidence is consistent with the proposed polymer repeat unit.
| Assignment |
Ph3AsBr2 |
Histamine |
Polymer |
Ph3SbCl2 |
Polymer |
| CH ip st |
3064 |
|
3051 |
|
3083 |
| CH st |
|
3083 |
3083 |
3060 |
3048 |
| CH2 asym st |
|
2984 |
2991 |
|
2994 |
| N-H ring st |
|
2968 |
|
|
|
| CH2 sym st |
|
28,922,810 |
####### |
|
28,962,850 |
| ring st |
|
162,515,251,474 |
####### |
|
162,615,301,478 |
| NH2 scissoring |
|
1596 |
|
|
|
| C=C st |
1465 |
|
1461 |
1465 |
1478 |
| M-Ph st |
1438 |
|
1438 |
1440 |
1432 |
| C=C ip wag |
1362 |
|
1360 |
1360 |
1359 |
| CH2 wag |
|
1346 |
1346 |
|
1332 |
| CH2 twist |
|
1294 |
1295 |
|
1305 |
| M-N |
|
|
1231 |
|
1247 |
| C-C st |
11,781,152 |
|
####### |
11,781,152 |
11,821,155 |
| M-Ph sym st |
1050 |
|
1053 |
1048 |
1068 |
| CH ip bend |
|
1090 |
1086 |
|
1094 |
| Sket st |
|
1030 |
1023 |
|
1021 |
| Ring vib |
997 |
|
997 |
997 |
997 |
| Ring breath |
|
960 |
950 |
|
962 |
| Ip ring |
|
851 |
860 |
|
862 |
| Skeletal op wag |
|
781 |
794 |
|
805 |
| Sym op bend |
736 |
|
741 |
732 |
725 |
| Aryl op bend* |
690 |
|
690 |
690 |
687 |
*Asymmetric out-of-plane bending of ring hydrogens for only mono-, meta, or 1,3,5 substitution.
Table 2. Assigned infrared spectral peaks found in the spectra for the monomers histamine, triphenylarsenic dibromide, and triphenylantimony dichloride and the polymer products of triphenylarsenic dibromide and triphenylantimony dichloride with histamine. Band locations are given in cm-1.
Proton NMR
NMR was carried out on the products refer Table 3. All values are given in ppm. Assigned bands correspond to the numbers given in the Figure 3. Expected bands are present for both the two monomers and resulting polymers. Assignments are consistent with those reported in literature.
| Assignment |
Histamine |
Ph3AsBr2 |
Polymer |
Ph3SbBr2 |
Polymer |
| C-H Ring,Ortho |
|
8.2 |
8.16 |
8.2 |
8.2 |
| C-H Ring, Meta, Para |
|
7.70,7.60 |
7.71,7.60 |
7.70,7.60 |
7.68,7.49 |
| 1 |
11.6 |
|
|
|
|
| 5 |
7.4 |
|
6.9 |
|
7.38 |
| 6 |
3.3 |
|
3.3 |
|
3.12 |
| 7 |
2.99 |
|
2.99 |
|
3.01 |
| 8 |
1.3 |
|
2.48 |
|
2.48 |
Table 3. NMR bands found for the monomers histamine, triphenylarsenic dibromide and triphenylantimony dichloride and the polymeric products from triphenylarsenic dibromide and triphenylantimony dichloride with histamine. Assignment band numbers are given in the Figure 3 for histamine.
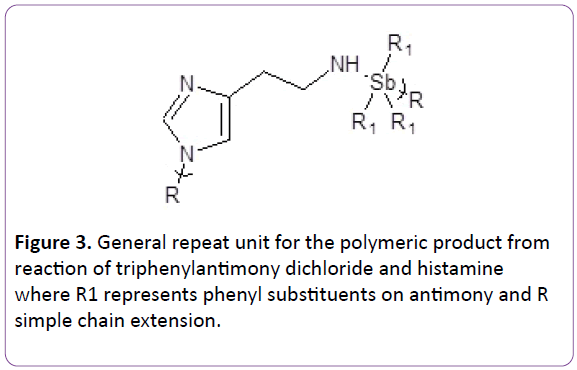
Figure 3: General repeat unit for the polymeric product from reaction of triphenylantimony dichloride and histamine where R1 represents phenyl substituents on antimony and R simple chain extension.
The band associated with the ring N-H is missing since it is now N-M. The band associated with the amine is shifted as expected from about 1.3 to about 2.5.
MALDI MS analysis
MALDI MS was developed to investigate non-volatile solids [38-41] because the polymers are not soluble in suitable volatile liquids to allow intimate association between the matrix and polymer sample traditional MALDI MS is not possible. We have been investigating the use of an alternative approach that focuses on the fragments [14-19]. This technique has been reviewed [42-47]. MALDI analysis was performed on the polymers.
Figure 4 contains a portion of the MALDI MS for the triphenylarsenic polymers and Table 4 contains assignments for the major ion fragments over the entire range studied (to 1200 Da). All ion fragments are described in terms of Da.
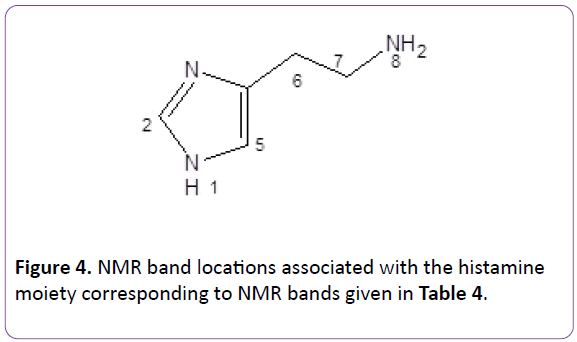
Figure 4: NMR band locations associated with the histamine moiety corresponding to NMR bands given in Table 4.
| Ion Frag., Da |
(Tentative) Assignment |
Ion Frag., Da |
(Tentative) Assignment |
| 542 |
U+HA,Na |
758 |
2U-Ph |
| 589 |
U+PhAs, Na |
781 |
2U+N-Ph |
| 645 |
U+Ph2As |
846 |
2U+NH |
| 668 |
U+Ph2As,Na |
871 |
2U,NH,Na |
| 721 |
U+Ph3As |
1046 |
2U+Ph2Sb-NH |
| 736 |
U+Ph3As,N |
|
|
Table 4. Ion fragment clusters derived from the product of triphenylarsenic dibromide and histamine.
Abbreviations are used to describe the assignments. These are U for unit, 2U for two repeat units, N for nitrogen, HA, for histamine minus two protons, Na for sodium and Ph for phenyl. These abbreviations are also employed for assignments for the other polymers here are loss of phenyl groups. This is common because of the sensitivity of alkyl-metal linkages to the radiation employed in MALDI MS. This loss is believed to occur only at sites of chain scission. The mildness of MALDI MS is shown by the lack of fragmentation of histamine.
Ion fragment clusters derived from the product of triphenylantinomy dichloride and histamine are given in Table 5. Fragments to over three units are found.
| Ion Frag. Cluster, Da |
(Tentative) Assignment |
Ion Frag. Cluster, Da |
(Tentative) Assignment |
| 540 |
U+HA-2NH |
776 |
2U+PhSb |
| 575 |
U+HA |
890 |
2U+NH,Na-Ph |
| 621 |
2U,NH,Na-2Ph |
1106 |
2U+PhSb |
| 656 |
U+PhSb |
1370 |
3U-NH |
| 736 |
U+Ph2Sb |
1490 |
3U+HA |
Table 5. Major ion fragment clusters derived from the MALDI MS for triphenylantimony dichloride and histamine polymer product obtained over the mass range of 500 to 2000 Da. They are described as ion clusters because they contain ions from various antimony isotopes.
Unlike bismuth and arsenic which have no natural occurring isotopes, antimony contains two isotopes. This allows isotopic matches to be made. Table 6 contains matches for ion fragment clusters containing one, two and three antimony atoms. The agreement is reasonable to that shown as “Known”, appearing in the two most left-hand columns and is consistent with the present of one, two, and three antimony atoms within these ion fragment clusters. The right hand columns contain the particular ion fragment location (Da) and the percentage relative abundance. The entry above this is the proposed structure for the particular ion fragment cluster.
| Known for Sb |
U+PA-CO2,O |
| 121 |
100 |
608 |
100 |
| 123 |
75 |
610 |
74 |
| Known for 2Sb |
2U+Na, NH-2Ph |
| 242 |
67 |
888 |
68 |
| 244 |
100 |
890 |
100 |
| 246 |
37 |
892 |
39 |
| Known for 3Sb |
2U+PhSb |
| 363 |
45 |
1104 |
52 |
| 365 |
100 |
1106 |
100 |
| 367 |
75 |
1108 |
71 |
| 369 |
19 |
1110 |
19 |
Table 6. Isotopic abundance matches for antimony-containing ion fragments derived from the MALDI MS of the product of triphenylantimony dichloride and histamine where the ion fragments contain one (top), two (center) and three (bottom) antimony atoms in the observed ion fragment. The first column gives the known mass (Da) for antimony followed by the percentage relative abundance for that particular isotope. The second two columns contain the found mass for the particular ion fragment whose structural assignment is given followed by the experimentally found relative abundance mass associated with that antimony-containing ion fragment.
Table 7 contains the ion fragment assignments for the product from triphenyl bismuth dichloride and histamine. Ion fragments to over three units are found.
As in other studies, chain scission occurs primarily at the heteroatoms as shown in Figures 5 and 6.
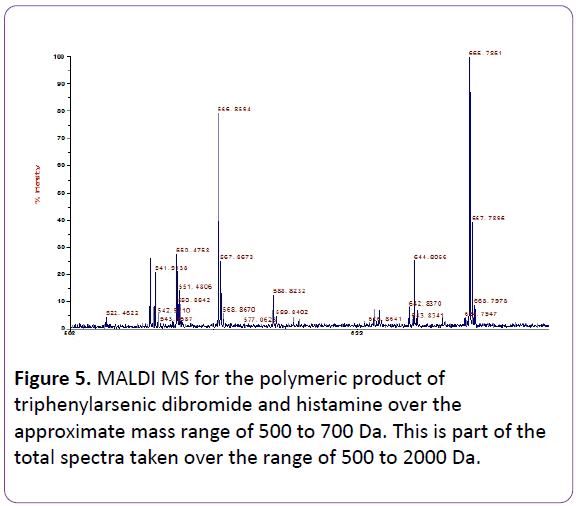
Figure 5: MALDI MS for the polymeric product of triphenylarsenic dibromide and histamine over the approximate mass range of 500 to 700 Da. This is part of the total spectra taken over the range of 500 to 2000 Da.
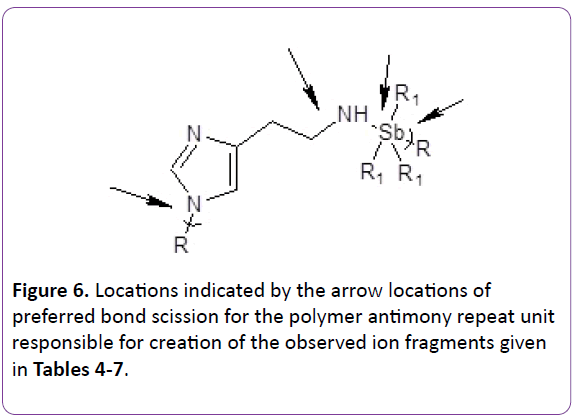
Figure 6: Locations indicated by the arrow locations of preferred bond scission for the polymer antimony repeat unit responsible for creation of the observed ion fragments given in Tables 4-7.
| Ion Frag. Da |
(Tenative) Assignment |
Ion Frag., Da |
(Tentative) Assignment |
| 532 |
U-NH |
935 |
U+Ph2Bi, Na |
| 550 |
U |
1083 |
2U-NH |
| 627 |
U+HA-2NH |
1156 |
2U+HA,Na-Ph |
| 658 |
U+HA |
1207 |
2U+HA |
| 681 |
U+HA,Na |
1230 |
2U+HA,Na |
| 835 |
U+PhBi |
1429 |
3U+HA |
| 912 |
U+Ph2Bi |
|
|
Table 7. Major ion fragments derived from the MALDI MS of the product of triphenylbismuth dichloride and histamine over the approximate range of 500 to 2000 Da. The first column contains the mass of the particular ion fragment and the second column contains the tentative structural assignment for that ion fragment.
Tumor analysis
Cancer is the leading cause of death world-wide. In this study we used a broad range of solid cancer cell lines (Table 8).
| NCI Designation |
Strain Number |
Species |
Tumor Origin |
Histological Type |
| PC-3 |
3465 |
Human |
Prostate |
Carcinoma |
| HT-29 |
1507 |
Human |
Recto-sigmoid colon |
Adenocarcinoma |
| MDA MB-231 |
7233 |
Human |
Pleural effusion-breast |
Adenocarcinoma |
| MCF-7 |
7259 |
Human |
Pleural effusion-breast |
Adenocarcinoma |
| WI-38 |
ATCC CCL-75 |
Human |
Normal embryonic lung |
Fibroblast |
| NIH/3T3 |
CRL-1658 |
Mouse |
Embryo-continuous cell line; highly contact-inhibited cells |
Fibroblast |
| PANC-1 |
|
Human |
Epithelioid pancreatic cells |
Carcinoma |
| AsPC-1 |
|
Human |
Pancreatic cells |
Adenocarcinoma |
Table 8. Descriptions for the cell lines employed in the current study.
The polymer drugs are cytotoxic and cell death is by necrosis [45,48,49]. Anticancer activity is occurs with the intact polymer without polymer degradation [45,49,50]. This is in agreement with our observations that the polymers do not degrade in DMSO with half-chain lives, the time for the polymer chain length to halve, greater than 30 weeks [45,48,49].
Most organometallic compounds associate with polar solvents such as DMSO. This association can influence the observed biological results. We find that this is generally not the case [45-54] for polymers with similar structure to those reported here with the influence less than 20% [45,50].
Evaluation of the effectiveness for test material to inhibit cell growth is generally found using two measures. The first measures the amount required to reduce growth of a particular cell line. This concentration is often called effective concentration, EC. Thus the concentration of the test material inducing a response halfway between the baseline and maximum after a specified exposure time is called the 50% response concentration, EC50.
The two most widely used cell line standards are the NIH/3T3 (often referred to as simply 3T3 cells) and WI-38 cell lines. NIH/3T3 cells are mouse embryo fibroblast cells that are partially transformed cells. Unlike normal cells they are immortal but retain other normal cell characteristics. They are robust and more easily maintained compared to normal cell lines.
The second standard cell line is WI-38 cells which are normal embryonic human lung fibroblast cells. Compared to NIH/3T3 cells, they are more fragile and difficult to maintain. Typically, they are preferred over the WI-38 cells because of ease of handling aided by an infinite life span.
In this study, the EC50 values for the NIH/3T3 and WI-38 cell lines give similar values for the cancer cell lines. When there is a difference, it is customary to employ WI-38 cell results [55].
EC50 values for the monomers and polymers are given in Table 9. Values for cisplatin are also given. Cisplatin, and related platinum drugs, is among the most widely employed anticancer drugs. It is highly toxic with many unwanted side effects. Many of the tested compounds have low toxicities towards the tested cell lines. Histamine shows no ability to inhibit any of the cell lines to the concentration limits employed.
| Sample |
WI-38 |
3T3 |
AsPC-1 |
PANC-1 |
| Ph3AsBr2 |
>32000 |
>32000 |
390(11) |
>32000 |
| Ph3As/HA |
>32000 |
>32000 |
>32000 |
10(0.97) |
| Ph3SbCl2 |
3500(34) |
3600(21) |
>32000 |
6500(18) |
| Ph3Sb/HA |
1.12(0.11) |
2.48(0.24) |
8.5(0.88) |
7.9(0.83) |
| Ph3BiCl2 |
790(90) |
600(16) |
3700(22) |
2100(10) |
| Ph3Bi/HA |
1.7(0.14) |
0.27(0.03) |
3.9(0.35) |
89(9.7) |
| Histamine(HA) |
>32000 |
>32000 |
>32000 |
>32000 |
| Cisplatin |
0.015(0.01) |
0.0023(.005) |
0.0023(.005) |
0.0035(0.005) |
| Sample |
7233/MDA |
7259/MCF-7 |
1507/HT-29 |
3465/PC-3 |
| Ph3AsBr2 |
21.(1.0) |
24.(2.1) |
16.(1.2) |
20.(1.7) |
| Ph3As/HA |
>32000 |
>32000 |
>32000 |
>32000 |
| Ph3SbCl2 |
12.4(1.1) |
130(11) |
33.(3.1) |
38(4) |
| Ph3Sb/HA |
2.2(0.26) |
21(2.7) |
1.5(0.22) |
3.8(0.29) |
| Ph3BiCl2 |
1.4(0.2) |
1.6(0.21) |
2.2(0.16) |
2.4(0.16) |
| Ph3Bi/HA |
0.32(0.03) |
3.2(0.29) |
4.7(0.41) |
4.1(0.42) |
| HA |
>32000 |
>32000 |
>32000 |
>32000 |
| Cisplatin |
0.0044(0.004) |
0.0029(0.002) |
0.0041(0.003) |
0.0057(0.003) |
Note:Values given in ( ) are standard deviations for each set of measurements.
Table 9. EC50 Concentrations (micrograms/mL) found for the tested compounds as a function of the particular cell lines whose description is given in Table 8.
All of the polymers inhibiting one or more cancer cell lines. Pancreatic cancer is one of our main focuses because it does not have a “cure” once it metastasizes. In the USA nearly 32,000 individuals die each year from pancreatic cancer. The human prostate cancer cell lines employed in the current study are AsPC-1, an adenocarcinoma pancreatic cell line responsible for about 80% of the human pancreatic cancers and PANC-1an epithelioid carcinoma pancreatic cell line which that appears in 10% of human pancreatic cancers. The bismuth and antimony show inhibition of both pancreatic cancer cell lines.
The breast cancer cell lines represent a matched pair of cell lines. The MDA-MB-231 (strain number 7233) cells are estrogenindependent, estrogen receptor negative while the MCF-7 (strain line 7259) cells are estrogen receptor (ER) positive. In some studies there existed a major difference in the polymer’s ability to inhibit the two breast cell lines [45,48,55-57]. When the Lewis base in the polymer possessed a O-Phenylene group such as found in some hormone treatments, the polymer showed greater ability to inhibit the MDA-MB-231 estrogenindependent cells possibility because the MCF-7 cells reacted with the polymers removing them from inhibiting the MCF-7 cells. Here, the arsenic product shows little ability to inhibit either breast cell line, but both the antimony and bismuth show good ability to inhibit both cell lines, favoring the inhibition of the estrogen independent MDA-MB-231 cell line at about a tenfold lower concentration compared to the estrogen receptor positive MCF-7 cell line. Histamine does not possess an OPhenylene structure so it is currently not known why this difference exists.
Both of the antimony and bismuth polymers show good ability to inhibit the 1507 colon and 3465 prostate cell lines. Colorectal cancer is also referred to by other names such as rectal cancer, colon cancer, colorectal adenocarcinoma, and bowel cancer. The focus is on treating uncontrolled cell growth, cancer, in the colon or rectum or related area of the body. These various cancers are genetically the same cancer. Cancers confined within the colon wall are generally curable with surgery while cancer that has spread throughout the body is typically not curable and management is by chemotherapy and improving the quality of life. Colorectal cancer is the third most diagnosed cancer worldwide being most common in developed countries. According to the American Cancer Society for 2014 about 137,000 people will be diagnosed with colorectal cancer with about 50,000 predicted to die of the disease in the USA. The HT-29 cell line is the most widely employed colon cancer cell line for studying a compounds ability to inhibit cell growth.
Based on EC50 results, the antimony and bismuth polymers show a good ability to inhibit most of the cancer cell lines while the arsenic polymer exhibits little ability to inhibit any of the cancer cell lines.
The second measure, often called the chemotherapeutic index, CI, of the potential use of compounds as anticancer materials is the concentration of drug necessary to inhibit the standard cells compared to the concentration of drug necessary to inhibit the growth of the test standard cell line, here WI-38 and NIH/3T3 cells. Here the focus is on the WI-38 results since these are preferred compared to the NIH/3T3 results. The CI50 is simply the ratio of the EC50 for the standard cell line WI-38 cells divided by the EC50 for the particular test cell.
CI50 values derived from values given in Table 9 are given in Table 10. (Please note that the experimental concentration upper limit is 32000 nanograms/mL is found in some cases. When samples exceed this limit they are cited as >32000 and values of >32000 divided by some value are entered as > followed by a number.
| Sample |
EC50 WI-38/ |
EC50 WI-38/ |
EC50 WI-38/ |
EC50 WI-38/ |
EC50 WI-38/ |
EC50 WI-38/ |
| |
EC50AsPC-1 |
EC50PANC-1 |
EC50HT-29 |
EC50PC-3 |
EC50MCF |
EC50MDA |
| Ph3As/HA |
1 |
>3200 |
1 |
1 |
1 |
1 |
| Ph3Sb/HA |
0.13 |
0.14 |
0.73 |
0.29 |
0.052 |
0.5 |
| Ph3Bi/HA |
0.43 |
0.019 |
0.36 |
0.41 |
0.53 |
5.3 |
| Cisplatin |
8.2 |
5.4 |
4.3 |
6.6 |
4 |
3.3 |
Table 10. CI50 values calculated from the EC50 data given in Table 9 as simple ratios of the EC50 for the standard value obtained from the standard WI-38 cell line divided by the observed EC50 for the particular cancer cell line type. The samples are all polymeric with the exception of cisplatin.
CI50 values of two and greater are generally considered significant. There is a single instance where a CI50 value is greater than 2. This is the large CI50 value >3200 found for the arsenic polymer against the PANC-1 pancreatic cancer cell line. It is a result of the polymer having a very low toxicity towards the standard WI-38 cell line coupled with a decent toxicity towards the PANC-1 pancreatic cancer cell lines. This high CI50 value is significant with further testing signaled for this polymer. In all cases, once inhibition begins, there is a steep slope in the inhibition verses concentration curve with total inhibition the end result.
Summary
Group VA polyamines have been synthesized in low yield with moderate to high molecular weight employing commercially available reactants, with the exception of the triphenylarsenic dibromide. The interfacial polycondensation is employed industrially to synthesize aramid fibers and polycarbonates [58,59]. Thus, there is a straightforward pathway production of the polymers from small to large amounts. Infrared spectroscopy shows the formation of new bands characteristic of the formation of M-N bonds. MALDI MS shows formation of ion fragment clusters about three units long with isotopic abundance results for the antimony polymers showing the presence of antimony in the ion fragment clusters. The absence of the histamine ring proton in the NMR spectra is consistent with the formation of the polyamine structure. Tumor analysis shows that the arsenic and bismuth polymers exhibit good cancer cell inhibition of all of the tested cancer cell lines. The arsenic polymer shows among the best CI50 values thus far found against the PANC-1 pancreatic cancer cell line. It will be considered for live animal testing.
This study is part of an ongoing effort whereby various Lewis acids and bases are combined in an effort to more fully understand which combinations are best at inhibiting cancer cell lines. While pancreatic and breast cancers are particular focal cancer, all of the cancers are of concern. Until a few years ago, there were no good agents that could inhibit pancreatic cancer cell lines so such studies give us and other researchers added evidence with respect to which compounds might be important to follow up with live animal studies. The current study gives one such combination worthy of additional study.
Histamine is one of the initial diamine Lewis bases studied by us and its ability to show some inhibition of all of the cell lines indicates that further work with diamine-containing reactants is warranted.
References
- Eischenbraich C (2006) Organometallic. Wiley-VCH, Weinheim, Germany.
- Grund SC, Hamusch K, Wolf H (2008) Arsenic and Arsenic Compounds. Wiley-VCH, Weinheim, Germany.
- Suzuki H, Matano Y (2001) Organobismuth Chemistry. Elsevier.
- Carraher C (1985) Biological activities and medical applications of metal-containing macromolecules. Plenum, NY.
- Naka K, Chujo Y (2007) Organic-inorganic hybrid polymers employing characteristics of hetero atoms. Kanaku to Kogyo 60: 520-523.
- Carraher C (2008) Antimony-containing polymers. J Polym Mater 25: 35-50.
- Karak N, Maiti S, Das S, Dey SH (2003) Antimony polymers part 5. Biological activity. J Polym Mater 20:237-242.
- Karak N, Maiti S (1996) Antimony containing polymers. J Polym Mater 13:179-190.
- Karak N, Maiti S (1999) Antimony polymers. part 2. Physical, chemical, and thermal properties. Angew Makromol Chemie 265: 5-12.
- Carraher C, Hedlund L (1980) Synthesis and characterization of antimony (V) polyoximes. J Macromol SciChem A14: 713-728.
- Carraher C, Venable W, Blaxall HS, Sheats JE (1980) Synthesis and characterization of antimony (V)-polycobalticinium exters. J Macromol Sci Chem A 14: 571-579.
- Carraher C, Blaxall HS (1979) Synthesis and solution characterization of antimony polyesters. Angew Makromol Chemie 83:37-45.
- Carraher C, Naas M, Giron DJ, Cerutis DR (1983) Structural and biological characterization of antimony V polyamines. J Macromol SciChem A19: 1101-1120.
- Sabir T, Carraher C (2006) Synthesis of triphenylantimony and triphenylbismuth-containing polyether amines containing acyclovir. J Polym Mater 4: 403-413.
- Carraher C, Truong N, Roner MR, Moric A, Trang N (2013) Synthesis of organoarsenic, organoantimony, and organobismuth poly(ether esters) from reaction with glycyrrhetinic acid and their preliminary activity against pancreatic cancer cell lines. JCAMS 1: 134-150.
- Carraher C, Roner MR, Thibodeau R, Moric-Johnson A (2014) Synthesis, structural characterization and preliminary cancer cell study results for poly(amine esters) derived from triphenyl-group VA organometallics and norfloxacin. Inorganic Chim Acta 423: 123-131.
- Carraher C, Roner MR, Dorestant J, Moric-Johnson A, Al-Huniti M (2015) Group VA poly(amine esters) containing the antibacterial ampicillin. J Inorg Organomet Polym 25: 400-410.
- Carraher C, Roner MR, Pham N, Moric-Johnson A (2014) Group VA polyesters containing thiodiglycolic acid-synthesis and preliminary cancer activity. J Macromol Sci A Pure Appl Chem 51: 547-556.
- Carraher C, Roner MR, Ayoub M, Pham N, Moric-Johnson A (2015) Synthesis and preliminary cancer activity of chelidonic acid polyesters containing triphenylarsenic, triphenylantimony, and triphenylbismuth moiety. Internat J Polym Mat Polym Biomat 64: 311-319.
- Ratnala VR, Hulsbergen F, de Groot H, de Grip WJ (2003) Analysis of histamine and modeling of ligand-receptor interactions in the histamine H(1) receptor for Magic Angle Spinning NMR studies. Inflamm Res 52: 417-423.
- Reite OB (1972) Comparative physiology of histamine. Physiol Rev 52:778-819.
- Green JP (1970) Does histamine interacts with cholinergic neurones in its cataleptogenic action in the rat. Handbook Neurochem 4: 221-250.
- Hesas HJ (1982) Annual Reports in Medicinal Chemistry, Technologies in Drug Discovery and Validation, Elsevier, NY, NY.
- Modder JF, Vrieze K, Spek AL, Challa G, van Koten G (1992) Stereoregular coordination polymers formed on binding of peptide-based polydentate ligands to silver(I) and copper(I). X-ray structure of ([Ag{N-[N-((5-methyl-2-thienyl)methylidene)-L-methionyl]histamine}]+[O3SCF3]-.MeOH) and a solution structure study. Inorg Chem 31: 1238-1247.
- Jeong H, Lee BJ, Cho WJ, Ha SC (2000) Polymeric Langmuir–Blodgett films containing imidazole-coordinated metal complexes. Polymer 41: 5525–5529.
- Min KS, Suh MP (2000) Self-assembly, structures, and magnetic properties of ladder-like copper (II) coordination polymers. J Solid State Chem 152: 183-190.
- Anderson DG, Lynn DM, Langer R (2003) Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed Engl 42: 3153–3158.
- Nelson TL, O’Sullivan C, Greene NT, Maynor M, Lavigme J (2006) Cross-Reactive Conjugated Polymers: Analyte-Specific Aggregative Response for Structurally Similar Diamines. J Amer Chem Soc 128: 5640-5641.
- Wong SY, Putnam D (2007) Overcoming limiting side reactions associated with an NHS-activated precursor of polymethacrylamide-based polymers. Bioconjugate Chem 18:970-982.
- Chen Y, Thorn MC, Christensen S, Versek S, Poe A, et al. (2010) Enhancement of anhydrous proton transport by supramolecular nanochannels in comb polymers. Nature Chemistry 2: 503-508.
- Brickleband N, Godfrey S, Lane H, Mcauliffe C, Pritchard R (1995) Synthesis and structural characterization of R3AsX2 compounds (R = Me, Ph, p-FC6 H4 or p-MeOC6H4; X2 = Br2, I2 or IBr); dependency of structure of R, X and the solvent of preparation. J Chem Soc Dalton Trans Inorg Chem 23: 3873-3876.
- Collado JA, Ramirez FJ, Raman J (1999) Infrared and Raman spectra of histamine?Nh4 and histamine?Nd4 monohydrochlorides. Journal of Raman Spectroscopy 30: 391-397.
- Collado JA, Ramirez FJ (2000) Vibrational spectra and assignments of histamine dication in the solid state and in solution. Journal of Raman Spectroscopy 31:925-931.
- Torreggiani A, Tamba M, Bonora S, Fini G (2003) Raman and IR study on copper binding of histamine. Biopolymers 72: 290-298.
- Ramirez FJ, Tunon I, Collado JA, Silla EJ (2003) Structural and Vibrational Study of the Tautomerism of Histamine Free-Base in Solution. J Amer Chem Soc 125: 2328-2340.
- Xerri B, Flament JP, Petitjean H, Berthomieu C, Bertomieu D (2009) Vibrational Modeling of Copper-Histamine Complexes: Metal-Ligand IR Modes Investigation. J Phy Chem B 113:15119-15127.
- Barber M, Bordoli RS, Sedwick RD, Tyler AN (1981) Fast atom bombardment of solids as an ion source in mass spectroscopy. Nature 293: 270-275.
- Liu LK, Busch KL, Cooks RG (1981) Matrix-assisted secondary ion mass spectra of biological compounds. Analyt Chem 53: 109-113.
- Tanaka K, Waki H, Ido Y, Akita S, Yoshida Y (1988) Protein and Polymer analyses up to m/z 100 000 by Laser Ionization Time-of flight Mass Spectrometry. Rapid Commun Mass Spectrom 2: 151-153.
- Karas M, Hillenkamp F (1988) Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem 60: 2299-2301.
- Carraher C, Barot G, Battin A (2009) Reactions between the matrix and ion fragments created from the MALDI MS or organotin-containing polymers. J Polym Mater 26:17-31.
- Carraher C, Sabir T, Carraher CL (2006) Fragmentation matrix assisted laser desorption/ionization mass spectrometry-basics. J Polym Mater 23:143-151.
- Carraher C, Sabir T, Carraher CL (2008) Inorganic Organometallic Macromolecules. Springer, New York.
- Carraher C, Roner MR (2014) Organotin polymers as anticancer and antiviral agents. J Organomet Chem 751: 67-82.
- Carraher C (2005) Organotin polymers. Wiley, Hoboken, NJ.
- Carraher C, Siegman-Louda D (2004) Organotin Macromolecules as Anticancer Drugs. Wiley, Hoboken, NJ.
- Carraher C, Roner MR, Shahi K, Barot G (2011) Structural Consideration in Designing Organotin Polyethers to Arrest the Growth of Breast Cancer Cells In Vitro. Materials 4: 801-815.
- Carraher C, Roner MR (2009) Antiviral Activity of Metal-Containing Polymers-Organotin and Cisplatin-Like Polymers. Materials 2: 1558
- Carraher C, Barot G, Nayak G, Roner MR (2013) Degradation of the Organotin Polyether Derived From Dibutyltin Dichloride and Hydroxyl-Capped Poly(ethylene Glycol) in Trypsin and Evaluation of Trypsin Activity Employing Light Scattering Photometry and Gel Electrophoresis. JCAMS 1:1-6.
- Carraher C, Barot G, Shahi K, Roner MR (2013) Influence of DMSO on the Inhibition of Various cancer Cells by Water-Soluble Organotin Poly(ethers). JCAMS 1:294-304.
- Ohtaki H (1987) Structural studies on solvationi and complexation of metal ions in nonaqueous solutions. Pure Appl Chem 59: 1143-1150.
- Gjevig Jenson K, Onfelt A, Wallin M, Lidumas O, Andersen O (1991) Effects of organotin compounds on mitosis, spindel structure, toxicity, and in vitro microtubule assemble. Mutagenessis 6:409-416.
- Corriu R, Dabosi G, Martineau M (1980) The nature of the interactioni of nucleophiles such as HMPT, DMSO, DMF and Ph3PO with triorganohalo-silanes, -germanes, and -stannanes and organophosphorus compounds. Mechanism of nucleophile induced racmization and substitution at metal. J Organomet Chem 186: 25-37.
- Ekwall B, Silano V, Paganuzzi-Stammati A, Zucco F (1990) Toxicity tests with mammalian cell cultures in short-term toxicity tests for non-genotoxic effects. Wiley, NY.
- Carraher C, Roner MR, Shahi K, Moric-Johnson A, Miller L (2015) Control of Breast Cancer Using Organotin Polymers. Internat J Poly Mater 64: 800-814.
- Barot G, Roner MR, Naoshima Y, Nago K, Shahi K (2009) Organotin Polyethers Derived from Hydroquinone and Substituted Hydroquinones. J Inorg Organometal Polym 19:12-27.
- Carraher C, Roner MR, Shahi K, Ashida Y, Barot G (2008) Synthesis and Initial Cell Line results of Organotin Polyethers containing Diethylstilbestrol. J Inorg Organometal Polym 18: 180-188.
- Carraher C (2017) Introduction to Polymer Chemistry. Taylor and Francis, NY, NY.
- Carraher C (2018) Polymer Chemistry. Taylor and Francis, NY, NY.