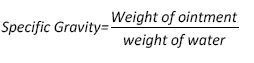Keywords
Lawsonia, Drug content, Permeability, Wound healing, Spreadability
Introduction
Natural product and their derivatives contribute almost 50% of all the drugs being used in clinical world. In a number of societies traditional medicines and allopathic treatment is going side by side. In present era of medicine, plants and their extracts are getting attention by the researchers to treat ailments all over the world in a traditional way [1]. Lawsonia inermis normally named as Henna is known as medically effective plant. Specific biophysical limits such as temperature 19-27 degree Centigrade and rainfall around 200-4200 mm respectively are optimum conditions for growth of Henna Plants [2]. The Lawson compound has found to have antibacterial effect, antifungal effect, antiviral effect, antiparasitic effect, molluscicidal effect, tuberculostatic effect, antifertility activity, analgesic activity, anti-inflammatory activity, cytotoxic activity, antisickling activity, abortifacient activity, enzymes inhibitory activity, nematicidal activity, anticoagulant effect, protein glycation inhibitory activity and potential to cure dysentery, liver disorders, baldness, headache [3]. Histopatholigical studies have revealed that a number of bioactive molecules like growth factors, cytokines, their receptors and matrix molecules play important role to regulate these steps of wound healing have been found in Lawsonia Inermis [4].
Henna leaves are used in crude form but it gives orange red color on skin and hair due to its active constituent Lawson. It is not possible to use it commonly. There was need to formulate such formulation which can be used easily to get its therapeutic effects in better way. In current study ointment formulated with minimum quantity of Lawson for its better effects to combat diseases. In the present study lawsonia ointment for topical application was formulated and characterized by drug content release, permeability, pH, viscosity, specific gravity. Ointment was also analyzed by measuring spreadabilty index. Ex vivo characterization effects on rat skin by Histopatholigical studies and in vivo evaluation of ointment in rats by using scratch and rash method was also carried out [5].
Material and Methods
Formulation of Ointment
Lawsonia Inermis was ordered commercially from Sigma Aldrich in active form Lawson. The six different formulations were prepared by alternating quantity of excipients and active drug for ointment purpose. White petrolatum was used 82% and yellow bees wax was used 4%. Quantity of Lawson was kept between 0.01 mg-0.1 mg. Optimized dosage form was finalized due to its excipients amount and also of its drug quantity. By maintaining excipients and changing its drug dose amount final dosage form was formulated and studied accordingly. Accurately weighed yellow bees wax was measured with help of weighing balance and was melted on 60°C. Lawsonia inermis was added portion wise in melted yellow bees wax and dissolved uniformly in a beaker. Then white petrolatum was measured with help of weighing balance and was melted on 60°C. Melted white petrolatum and all other ingredients (glycerol, paraffin oil, and cholesterol) were added in separate beaker one by one. All the components were melted and mixed geometrically. Mixture of pre-melted yellow bees wax and drug was added in it and dissolved at 60°C. After removing heat, it was geometrically mixed continuously with spatula to get uniform dosage form.
Methodology
pH
1 gram and 2.5 gram of each formulation was added in 100 ml of distilled water in a beaker respectively and stirred at 60°C-70°C for 1 hour. The solution was cooled at room temperature. pH of each formulation solution was measured with the help of pH meter [6,7].
Viscosity
10 g of each formulation was taken in separate beakers respectively. Beakers were placed one by one in viscometer and spindles were adjusted S5, S6, and S7 respectively at 10 rpm. Spindles were allowed to move up to 3 minutes to get fix reading. Readings were calculated in centipoise and percentages [7].
Specific Gravity
A beaker was taken and after washing and drying it, weight was determined accurately using weighing balance. 50 ml water was added in beaker and weight was determined again. Beaker was washed and dried then filled it with 50 gram of formulated ointment. The weight of beaker containing ointments was determined accordingly [8].
The specific gravity of formulation was calculated using following formula:
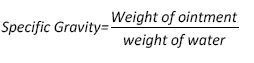
Measurement of Drug Content
UV absorbance for all formulations was calculated using UV spectrophotometer double beam7. The drug content of each formulation was calculated by comparing their UV absorbance value at pH 5.5 and 7.8 with calibration curve 1 and 2. Two calibration curves were made between UV absorbance value and concentration of active drug at pH 5.5 and 7.8 respectively with the help of Microsoft Excel. The formula produced by each calibration curve was used for calculation of drug content according to both curves respectively.
x=y – b/x
y=Absorbance
m=Slope
x=Concentration
b=y intercept
Calculation of Permeability
UV absorbance of samples was calculated by UV meter wavelength of 452 nm according to Verma et al. Permeability of all the samples was calculated by using software DDSOLVER. The permeability of each formulation was calculated by software at different pH according to both calibration curves. Percentage permeability of each formulation was noted on equal time intervals 1 hour, 2 hour, 3 hour, 4 hours and 5 hours respectively. Different kinetics models were applied on it to explain the drug release patterns. Zero order release kinetics, First order release kinetics, Higuchi release model, Hixon-Crowell model, Korsmeyer and Peppas release model (Table 1).
Table 1. Kinetics Models
| Model |
Purpose |
Formula |
| Zero order |
% age drug release verses time is linear |
C=kot |
| First order |
Log % age drug release verses time is linear |
og C=logCo – kt/2.303 |
| Higuchi model |
% age drug release versus square root of time is linear |
Q=k1t1/2 |
| Hixon – Crowell model |
Original drug mass, weight and mass, weight at time versus time is linear |
Qo1/3 – Qt1/3=kHCt |
| Korsmeyer and Peppas model |
|
Mt/M∞=kkptn |
Where, ko: Zero Order Kinetics; Co: Initial Drug Concentrations; k: First Order Rate Constant; k1: Constant Reflecting the Design Variable; kHC: Rate Constant of Equation; kkp: Rate Constant of Equation
Spreadability Test
3 grams of each formulation was measured and placed between two glass slides respectively. 1000 grams of weight was applied on glass slides. Weight was placed on a petri dish to provide uniform stress to get rid of air and uniform distribution of ointment thickness. 300 grams weight was tied to a hook; other end of that hook was tied to upper slide. Lower slide was separated with force and the time used to separate both slides was recorded. The time was calculated for each formulation similarly [9].
Spreadability for each formulation was calculated by using formula:
S=M × LT
Where S=Spreadability
M=Weight tied to the upper slide
L=Length of slides
T=Time recorded to separate slide
In vivo Studies of Wound Healing Activity
8 Healthy rats were taken from Animal house. Weight of rats was determined and a deep scratch of around 1 cm was given near ear of each mice. Active drug, simple base and all the six formulation was applied 4 times in a day on respective mouse. The procedure was carried out for 5 days and area of scratch on each mouse was measured daily. Percentage cure for each rat was calculated [9].
Results
pH
pH of all the formulations was calculated by two methods. In first method Formulation OLA-5 Gave highest value 6.02 and The Formulation OLA-3 gave the lowest value 5.079. Using second method Formulation OLA-5 gave the highest value 6.47 while lowest pH 5.107 was calculated in case of Formulation OLA-2 (Figure 1).
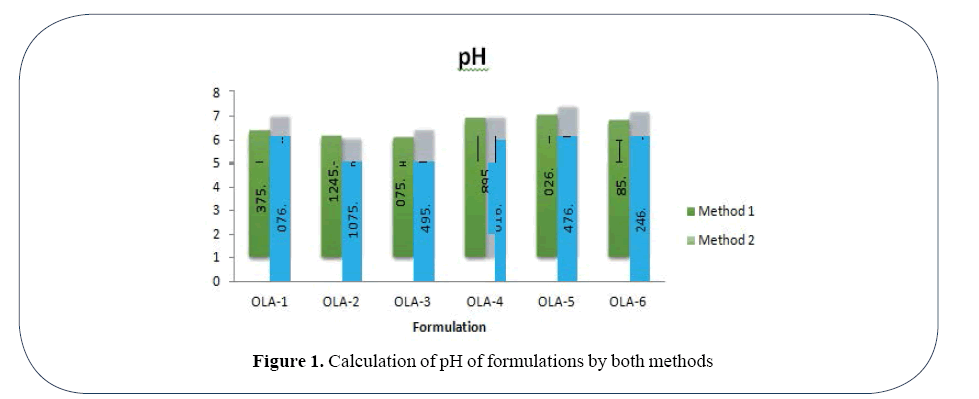
Figure 1. Calculation of pH of formulations by both methods
Viscosity
At spindle S5 Formulation OLA-1 gave the highest viscosity 28719 CP and OLA-4 gave the lowest viscosity 17.469. At spindle S6 Formulation OLA-6 gave the upper limit 19188 CP and Formulation OLA-5 gave the lowest. At spindle S7 Formulation OLA-5 gave the highest viscosity 5411.8 CP and Formulation OLA-6 gave the lowest viscosity 4622.7 (Figure 2).
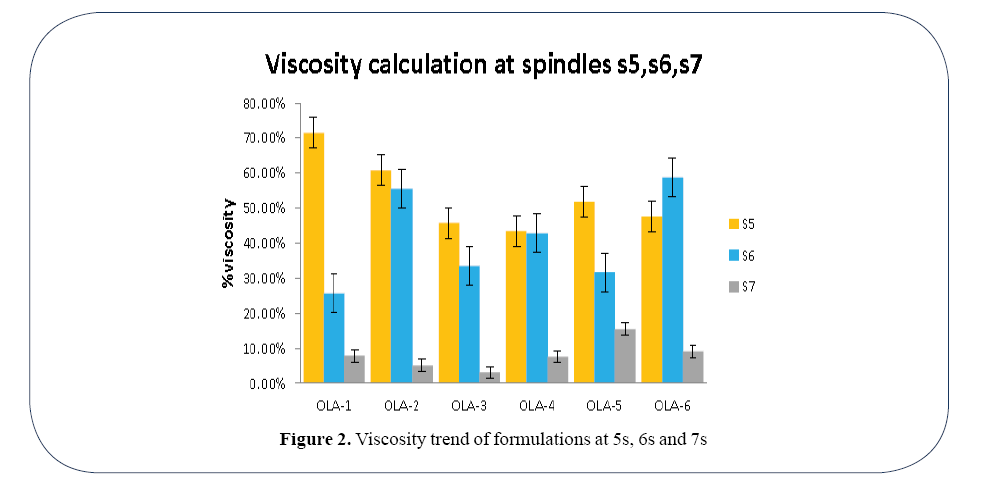
Figure 2. Viscosity trend of formulations at 5s, 6s and 7s
Specific Gravity
Specific gravity of formulations was found 0.87, 0.91, 0.81, 0.85, 0.86 and 0.91 respectively.
Highest specific gravity was 0.91 and lowest specific gravity was 0.085 as shown in Table 2.
Table 2. Percentage drug release in Buffer 7.8 by application of Zero order release kinetics, First order release kinetics, Higuchi release model, Hixon-Crowell model, Korsmeyer and Peppas release model
| Zero order (R2) |
First order (R2) |
Higuchi model (R2) |
Hixon Crowell (R2) |
Korsmeyer and Peppas |
| (R2) |
N |
| 0.9876 |
0.9881 |
0.8642 |
0.9825 |
0.9901 |
0.88 |
| 0.9864 |
0.987 |
0.8662 |
0.9807 |
0.9893 |
0.873 |
| 0.9978 |
0.9975 |
0.8191 |
0.9967 |
0.9971 |
1.021 |
| 0.8765 |
0.8721 |
0.9786 |
0.7472 |
0.9841 |
0.565 |
| 0.8891 |
0.8933 |
0.9241 |
0.8009 |
0.9325 |
0.615 |
| 0.9467 |
0.951 |
0.9094 |
0.9168 |
0.9642 |
0.723 |
The specific gravity variation of different ointments in demonstrated in (Figure 3).
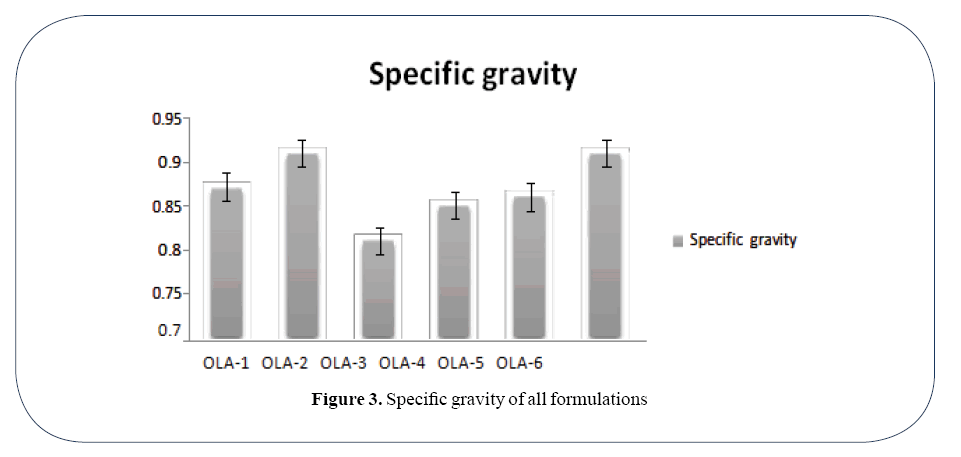
Figure 3. Specific gravity of all formulations
Drug Content
Using Calibration Curve 1 (Figure 4) Drug Content ranges from 0.764 (Formulation OLA-1) 1.716 (Formulation OLA-6) at pH 5.5. Similarly Drug content at pH 7.8 produced lower limit 0.029 (Formulation OLA-1) and higher limit of 0.715 (Formulation OLA-6). Using Calibration curve 2 (Figure 5) Drug Content ranges from 0.764 (Formulation OLA-1) 1.716 (Formulation OLA- 6) at pH 5.5. Similarly Drug content at pH 7.8 produced lower limit 0.029 (Formulation OLA-1) and higher limit of 0.715 (Formulation OLA-6) (Table 3).
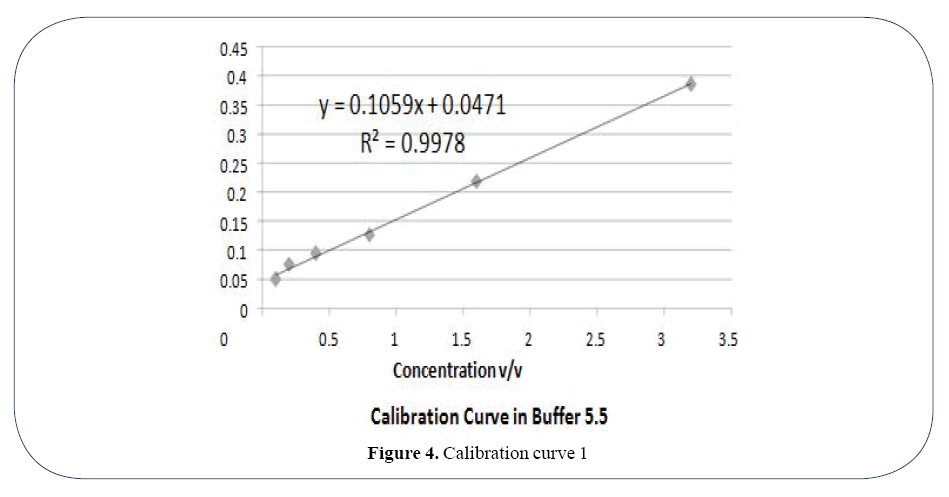
Figure 4. Calibration curve 1
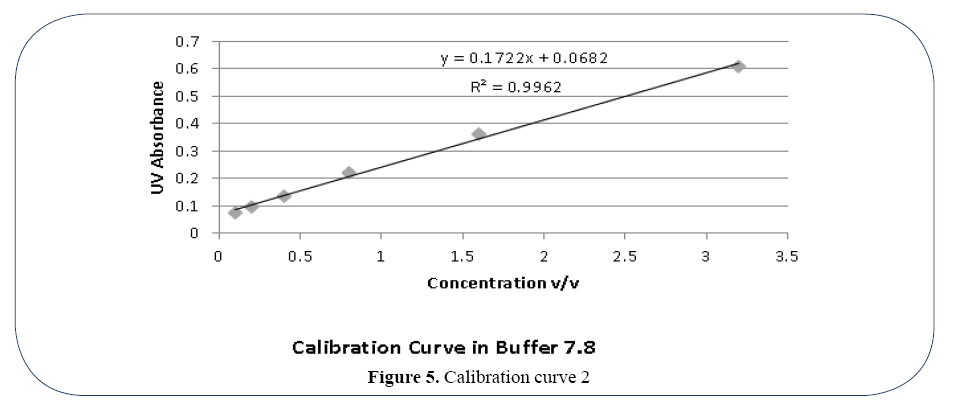
Figure 5. Calibration curve 2
Table 3. Drug Content of different formulations in buffers of different pH 5.5 and 7.8 using Curve 1
| Formulations |
Drug content in buffer pH 5.5 (g) |
Drug content in buffer pH 7.8 (g) |
| OLA-1 |
0.764 ± 0.008 |
0.029 ± 0.006 |
| OLA-2 |
0.84 ± 0.025 |
0.107 ± 0.02 |
| OLA-3 |
0.882 ± 0.015 |
0.519 ± 0.04 |
| OLA-4 |
0.921 ± 0.012 |
0.607 ± 0.026 |
| OLA-5 |
1.068 ± 0.029 |
0.627 ± 0.021 |
| OLA-6 |
1.176 ± 0.012 |
0.715 ± 0.04 |
Calculation of Permeability
In Buffer 7.8 the permeability ranges from 1.25435 d of OLA-1 to 12.36934 d of OLA-6 (Figure 6). In Buffer 5.5 the permeability ranges from 0.21957 d of OLA-1 to 8.19821 d of OLA-6 (Figure 7). Percentage drug release in buffer 5.5 and 7.8 using in vitro technique by application of Zero order release kinetics, First order release kinetics, Higuchi release model, Hixon-Crowell model, Korsmeyer and Peppas release model are enlisted in Tables 4 and 5 respectively.
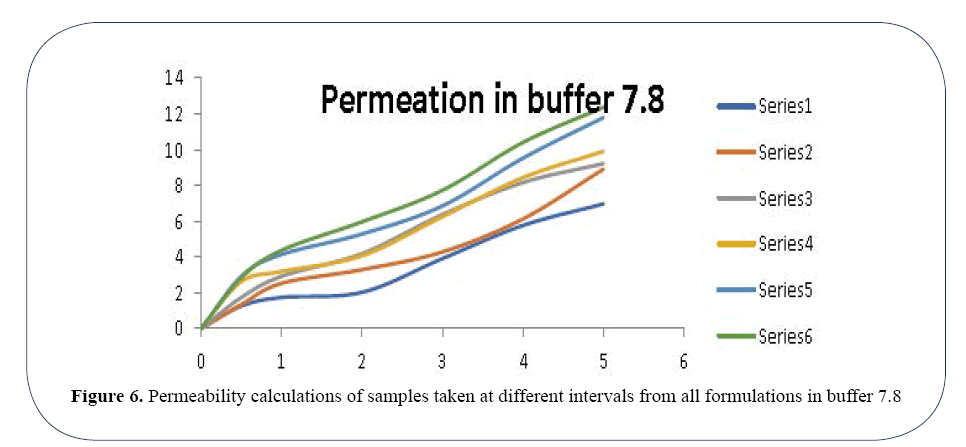
Figure 6. Permeability calculations of samples taken at different intervals from all formulations in buffer 7.8
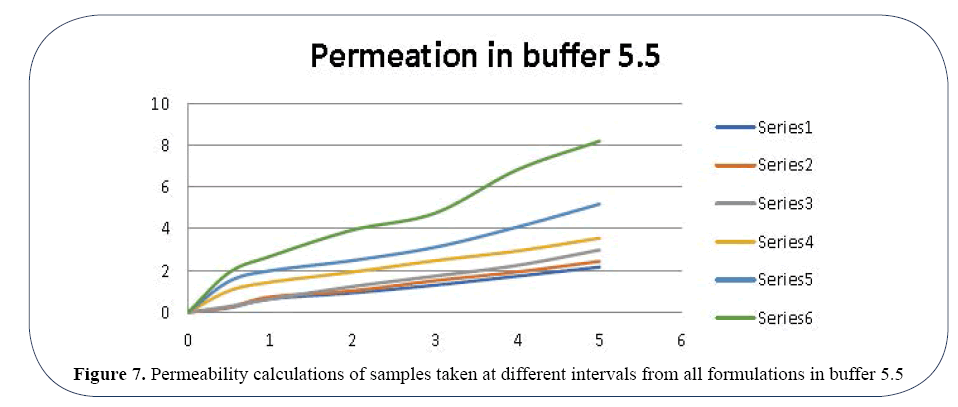
Figure 7. Permeability calculations of samples taken at different intervals from all formulations in buffer 5.5
Table 4. Drug Content of different formulations in buffers of different pH 5.5 and 7.8 using Curve 2
| Formulations |
Drug content in buffer pH 5.5 (g) |
Drug content in buffer pH 7.8 (g) |
| OLA-1 |
0.331 |
0.104 |
| OLA-2 |
0.377 |
0.058 |
| OLA-3 |
0.401 |
0.186 |
| OLA-4 |
0.424 |
0.238 |
| OLA-5 |
0.511 |
0.208 |
| OLA-6 |
0.575 |
0.302 |
Table 5. Percentage drug release in Buffer 5.5 by application of Zero order release kinetics, First order release kinetics, Higuchi release model, Hixon-Crowell model, Korsmeyer and Peppas release model
| Zero order (R2) |
First order (R2) |
Higuchi model (R2) |
Hixon Crowell (R2) |
Korsmeyer and Peppas |
| (R2) |
N |
| 0.9716 |
0.9705 |
0.7913 |
0.9591 |
0.9603 |
1.025 |
| 0.9605 |
0.9594 |
0.8402 |
0.9419 |
0.9438 |
0.955 |
| 0.9684 |
0.9738 |
0.9115 |
0.9561 |
0.992 |
0.763 |
| 0.9447 |
0.9495 |
0.8937 |
0.9145 |
0.9601 |
0.737 |
| 0.9336 |
0.9397 |
0.9045 |
0.8949 |
0.9576 |
0.704 |
| 0.9417 |
0.9417 |
0.9315 |
0.9112 |
0.9826 |
0.693 |
In vivo Study of Wound Healing Activity
The length of scar decreased from 1 cm on day 1 to 0.39 at day 6 in rat treated with active drug. The percentage cure in case of active drug was 71%. In case of rat treated with base only the healing rate was 2% with only length reduced by 0.02 cm on day 6. The length of scar reduced from 1 cm on day 1 to 0.43 at day 6 in mice treated with Formulation OLA-6 with percentage cure of 57%. The percentage cure in case of Formulation OLA-5 was 46% as the length of scar reduced from 1 cm on day 1 to 0.54 at day 6. Similarly in case of Formulation OLA-4, OLA-3, OLA-2 and OLA- 1 the length of scar decreased from 1 cm on day 1 to 0.61, 0.89, 0.91 and 0.94 at day 6 respectively. The healing potential was 39%, 11%, 9% and 6% accordingly (Figures 8 and 9).
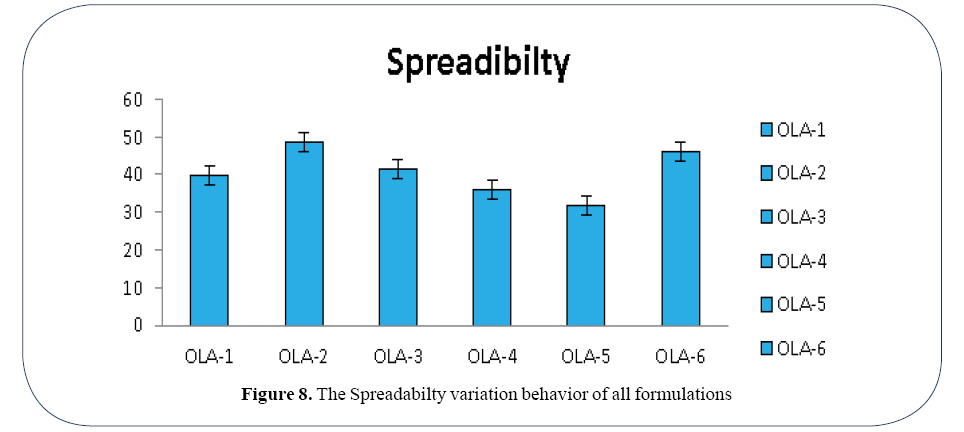
Figure 8. The Spreadabilty variation behavior of all formulations
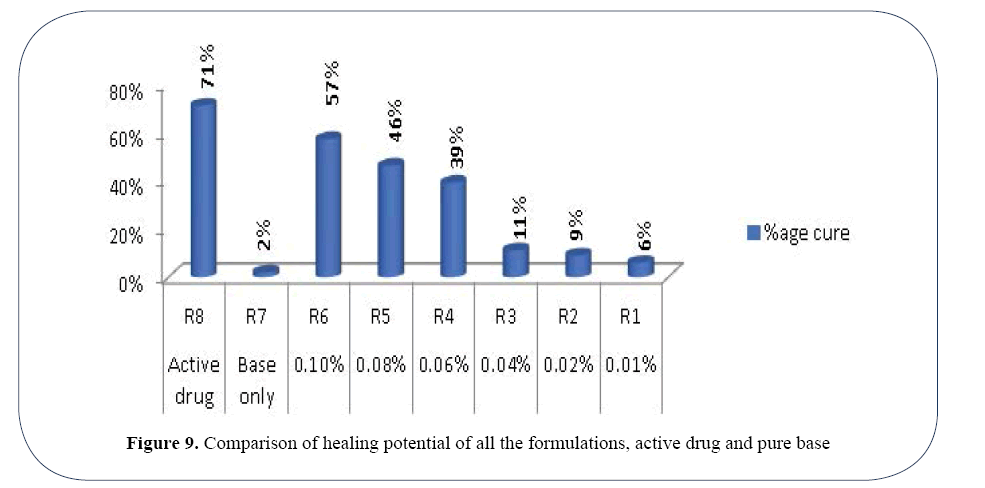
Figure 9. Comparison of healing potential of all the formulations, active drug and pure base
Discussion
Plants friendship with humans for the wellbeing of human is quite remarkable. Natural substances can support health and lighten illness is basically prime idea for the development of herbal medicines. Herbal remedies have become a trend now days as people are switching to herbal way of treatment. Western drugs also their origin in plant extract. According to Lochhead et al. specific gravity of stable and quick breaking topical skin composition ranges between 0.75-0.91, Formulation OLA-1, OLA-2 and OLA- 3 were in this ideal range [9]. Drug delivery of semisolid dosages depends on the rheological properties. Viscosity may directly control the diffusion rate of drug. Formulation OLA 6 was excellent for skin treatment as it pH is close to Skin pH. Aukunuru et al. showed that enhanced release of drugs has also been obtained by super saturation of the ointment base [10]. In this study, we effectively used a cosolvency technique to obtain the same results. Our results are in exact concordance with these findings. High skin permeability in case of ointment is indicator of higher drug release, provided that that drug partitioning into the skin is not an issue. Super saturation of the ointment base and co-solvency technique can be used to get increased release of drugs and optimal results. The FDMRA assay has been proven successful to optimize the formulation of ointment statically.
Nayak et al. used Ethanolic extract of Lawsonia inermis to study healing of wounds on rat‘s incision, excision and dead space wound models. It was found that extracted henna leaves having 71% efficacy exactly as in case of pure drug our study indicated that healing rate is 71% in case of pure drug [11]. According to Fahad, Natural henna is usually hypoallergenic and allergic reactions occurred in mixed types including black henna [12]. The patch test is the gold standard method for diagnosis of allergic contact dermatitis from henna, while the prevalence of the positive patch test in pure henna is 3 percent only, the directive of the European Community Cosmetics Directive, which allows the maximum concentration to be 6%. Nayeem and Karvekar, showed that the Spreadability of all gels was found to be between the ranges of 5.2 cm to 6.6 cm. In contrast our study showed that Spreadability of all formulation ranges between 39 cm to 48 cm.
Conclusion
This study was first time conducted in Pakistan for topical application of Lawsonia and showed promising results. The ointment formulation, characterization, spreadability, and in vivo studies indicate that it can be an excellent breakthrough in field of pharmaceutics. Additional biochemistry studies are required to sequester the active compound in authority for these pharmacological actions. Boosted wound shrinkage, improved skin breaking strength and antimicrobial potential suggest that future belongs to use of L. inermis in the managing of wound healing.
References
- Kamal M, Jawaid T. Pharmacological activities of Lawsonia inermis Linn: A review. International Journal of Biomedical Research. 2010;1(2):37-43.
- Orwa C, Mutua A, Kindt R, et al. Agroforestree database: A tree species reference and selection guide version 4.0. World Agroforestry Centre ICRAF, Nairobi, KE. 2009.
- Shivananda BN, Shivananda NB. Maniple Manuel of Clinical Biochemistry. Jaypee Brothers, Medical Publishers Pvt. Limited. 2007.
- Mehta D, Sharma AK. Trichosanthes dioica (Roxb.): A review on pharmacological update. Inventi. 2012;12:172.
- Najmuddin M, Shelar SA, Ali AS, et al. Formulation and in vitro evaluation of floating microspheres of ketoprofen prepared by emulsion solvent diffusion method. Int J Pharm Pharm Sci. 2010;2:13-17.
- Verma A, Singh S, Kaur R, et al. Formulation, optimization and evaluation of Clobetasol Propionate gel. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(4):666-674.
- Buhse L, Kolinski R, Westenberger B, et al. Topical drug classification. International Journal of Pharmaceutics. 2005;295(1):101-112.
- Nayeem N, Karvekar MD. Anti-microbial and anti-oxidant properties of the isolated compounds from the methanolic extract from the leaves of Tectona grandis. Journal of Basic and Clinical Pharmacy. 2011;2(4):163.
- Lochhead RY, Castaneda JY, Hemker WJ. Inventors: The BF Goodrich Company, assignee. Stable and quick-breaking topical skin compositions. United States patent US 5,004,598. 1991.
- Aukunuru JV, Ayalasomayajula SP, Kompella UB. Nanoparticle formulation enhances the delivery and activity of a vascular endothelial growth factor antisense oligonucleotide in human retinal pigment epithelial cells. Journal of Pharmacy and Pharmacology. 2003;55(9):1199-1206.
- Nayak BS, Isitor G, Davis EM, et al. The evidence based wound healing activity of Lawsonia inermis Linn. Phytotherapy Research. 2007;21(9):827-831.
- Fahad S, Hussain S, Saud S, et al. Exogenously applied plant growth regulators affect heat-stressed rice pollens. Journal of Agronomy and Crop Science. 2016;202(2):139-150.