Research Article - (2018) Volume 2, Issue 1
Paolo Lissoni*, Franco Rovelli, Fernando Brivio, Giusy Messina, Arianna Lissoni, Sonja Pensato and Giuseppe Di Fede
Institute of Biological Medicine, Milan, Italy
*Corresponding Author:
Paolo Lissoni
Institute of Biological Medicine, Milan, Italy
Tel: +39 02 5830 0445
E-mail: paolo.lissoni@gmx.com
Received date: March 09, 2018; Accepted date: March 09, 2018; Published date: March 26, 2018
Citation: Lissoni P, Rovelli F, Brivio F, Messina G, Lissoni A, et al. (2018) Five Year-Survivals with High-Dose Melatonin and Other Antitumor Pineal Hormones in Advanced Cancer Patients Eligible for the Only Palliative Therapy. Res J Oncol. Vol.2 No. 1: 2.
Copyright: © 2018 Lissoni P, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Cancer progression has appeared at least in part to be due to a deficiency of the mechanisms responsible for the natural antitumor immune response. Moreover, more recent studies have demonstrated that cancer-related immunosuppression does not depend only of alterations of immune cells themselves, but also on an altered neuroendocrine regulation of the antitumor immune response, which is mainly inhibited by the mu-opioid agonists, such as beta-endorphin, and stimulated by the pineal gland through at least three immunostimulating molecules, able to exert a direct antiproliferative anticancer activity without any important biological toxicity, consisting of the indole hormones melatonin (MLT) and the 5-methoxytryptamine (5-MTT), and of the beta-carboline pinealine (PNL). Finally, cancer progression has been shown to be constantly associated with a progressive decline in the endocrine function of the pineal gland, which could be involved in cancer dissemination itself. Then, the simple endocrine oncostatic pineal replacement therapy could counteract cancer growth and enhance the survival time, as suggested by preliminary clinical studies. On the basis, a pineal endocrine regimen was proposed in a group of untreatable advanced solid tumor patients, for whom no other effective standard anticancer therapy was available. The study included 212 patients, suffering from the most common tumor histotypes and eligible for the only best supportive care and with life expectancy less than 1 year. All pineal indoles were given orally at the time corresponding to that of their maximal circadian secretion, every day without interruption until disease progression. MLT was given at pharmacological doses (100 mg/day in the night period), while 5-MTT during the light period and PNL at the onset of the evening were administered at mild-pharmacological doses (5-MTT: 10 mg/day; PNL: 1 mg/day). A disease control (DC) was achieved in 111/212 (52%) patients, with an objective tumor regression in 16/212 (8%), irrespectively of tumor histotype. A 1-year and 5-year percentages of survival were achieved in 46% and 11%, respectively, and there were significantly higher in patients with DC than in the progressed ones. Finally, the evidence of normal pretreatment values of lymphocyte-to-monocyte ratio (LMR) and/or their normalization on therapy have appeared to be associated with most favorable clinical results. No biological toxicity occurred on pineal endocrine oncostatic treatment. This study shows that an endocrine substitutive therapy with the most known antitumor pineal hormones may represent a new non-toxic inexpensive anticancer therapy, which can improve the survival and control cancer growth also in patients for whom no other effective therapy is available, at least to improve their life. By concluding according to their results the palliative therapy of untreatable cancer patients for whom no other standard therapy available could be associated with a concomitant therapy with natural anticancer agents, namely the same pineal hormone.
Keywords
Lymphocyte-to-monocyte ratio; Melatonin; Metoxytryptamine; Pineal gland
Introduction
Each living organisms may generate both pro-tumoral and anti-tumoral events, from whose equilibrium depends the physiological growth of the normal cells until their apoptosisinduced death. The antitumor biological response, which is responsible for the natural resistance against cancer growth, would depend not only on immune factors, but also on the physiological psychoneuroendocrine regulation of the immune system, which may act by either stimulating or suppressing the antitumor immunity, as shown by the great number of researches in the area of the Psycho-neuro-endocrinoimmunology (PNEI) [1-3]. In particular, it has been shown that the opioid system may inhibit the anticancer immunity [4] by promoting the generation of regulatory T lymphocytes (T reg), which may suppress the antitumor immune response through the secretion of immunosuppressive cytokines, such as TGFbeta and IL-10 [5], and by inhibiting T helper-1 lymphocyte (TH1) and dendritic cells functions [6], with a following decline in the production of IL-2 and IL-12, respectively, that represent the main antitumor cytokines in humans [7,8]. On the contrary, the anticancer immunity has been proven to be stimulated by the pineal gland through the release of several indole hormones [9] and beta-carbolines [10], whose activity is connected with the brain cannabinergic system, by constituting a fundamental neuroendocrine functional axis [11]. More in detail, stress-induced promoting effect on cancer onset and development has appeared to be mediated by the opioid system, mainly through the release of mu-opioid agonists, such as the beta-endorphin, since it may be blocked by the concomitant administration of the mu-opoid antagonist naltrexone [4]. On the other hand, pleasure and spiritual expansion of mind may counteract tumor dissemination by activating the pineal-cannabinergic functional axis [12]. As far as the pineal activity is concerned, the main anticancer molecules are consisting of the indoles melatonin (MLT) and 5- methoxytryptamine (5-MTT) [9], and the beta-carboline pinealine [10], which exert their anticancer action by either directly inhibiting cancer cell proliferation, or stimulating the anticancer immunity, namely through the activation of TH1 lymphocytes and dendritic cells, with a following enhanced production of IL-2 and IL-12 [13,14]. The antitumor immunomodulating effects of MLT are mainly due to the stimulation of lymphocyte functions [15], whereas those played by 5-MTT, pinealine, as well as by cannabinoids, would mainly depend on an inhibition of macrophage-mediated immuno-inflammatory response [9,10], which has been proven to suppress the anticancer immunity [16,17]. Therefore, from a neuroimmune point is concerned, cancer growth may be considered as the consequence of an altered balance involving the main structures responsible for the neuroimmunomodulation of the immune responses, consisting of an enhanced brain opioid sistem activity in association with a concomitant diminished function of the pineal-cannabinergic system axis [18]. In fact, the progressive decline in the pineal function, namely consisting of a progressive lack of the nocturnal increase in MLT levels with a consequent disappearance of its physiological light/dark circadian rhythm [19], would represent the main cancer progression-related endocrine deficiency either in animals, or in humans [20,21]. Cancer-related pineal endocrine deficiency woul regard not only MLT, but probably the whole pineal endocrine activity, since pineal histological damages have been described in patients died from cancer [22]. However, despite it is known since more than 50 years that the pineal gland plays a fundamental role in the maintenance of the natural anticancer immunobiological resistance [9-11] and the complete absence of any biological toxicity exerted by the pineal indole and beta-carboline hormones [19], few clinical studies have been performed up to now with MLT alone or MLT in association with other antitumor pineal molecules to evaluate their efficacy in the treatment of advanced cancer patients, who failed to respond to the conventional chemotherapies and target therapies, at least in terms of palliative therapy. In any case, preliminary clinical studies have already shown that high-dose MLT alone may induced a stabilization of the neoplastic disease in a clinically relevant percentage of cancer patients, for whom no other standard anticancer therapy was available, and with life expectancy less than 6 months-1 year [23]. Moreover, it has been shown that the anticancer activity of MLT is a dose-dependent phenomenon, and may be further amplified by the concomitant administration of other antitumor pineal molecules, namely 5-MTT and pinealine [23-25]. However, many others natural anticancer strategies have been elaborated in the last year [26-28]. The present study reports the 5-year survival achieved by the pineal endocrine therapy with high-dose MLT plus 5-MTT plus pinealine in advanced cancer patients, for whom no other standard antitumor therapy was available, and its relation with the clinical response and the immune status by determining the lymphocyte-to-monocyte ratio (LMR), which has been proven to reflect and to synthetize the complex interaction between immunosuppressive and immunostimulatory events involved in the antitumor immunity [29].
Materials and Methods
Patient enrollment
The study included 212 advanced cancer patients, for whom no other standard anticancer therapy was available, then eligible for the only palliative treatment, who had a follow up of at least five years. Eligibility criteria were, as follows: histologically proven solid tumor, measurable lesions, metastatic or advanced neoplastic disease, no availability of conventional anticancer therapy because of lack of response to the previous standard treatments or poor clinical conditions unable to sustain a chemotherapeutic approach, no double tumor, and life expectancy less than 1 year.
Study plan
All pineal hormones were given orally. MLT was administered at 100 mg/day during the dark period of the day, according to its physiological circadian rhythm, generally halfhour before sleeping. 5-MTT was given at 10 mg/day during the light phase of the day, generally at 1.00 P.M. Finally, pinealine was administered at 1 mg/day in the evening, generally 3 hours prior to sleep. Moreover, the supportive care was planned according to a phythotherapeutic approach, by using plants, which have been proven to give some subjective benefits in previous clinical studies [30], namely Aloe, Myrrh, and Magnolia. The treatment with pineal hormones was continued without interruption until disease progression. In the presence of a clear subjective clinical benefit, pineal hormone therapy was still continued despite the progression of the neoplastic disease. The clinical characteristics of patients are reported in Table 1. The clinical response was evaluated according to WHO criteria by repeating the radiological investigations, including CT scan and NMR, before the onset of treatments and at 3-month intervals until disease progression. Moreover, the clinical response was correlated with LMR values, which were detected prior to therapy and at 1-month intervals. Normal values of LMR obtained in our laboratory (95% confidence limits) were greater than 2.1. Data were statistically analyzed by the chi-square test, the Student’s t test. Finally, the survival curves were made according to the Kaplan-Meyer method, and statistically assessed by the logrank test.
Table 1 Characteristics of 212 untreatable advanced cancer patients treated with pineal endocrine therapy (PET).
| Characteristics | n |
|---|---|
| M/F | 118/94 |
| Median age (years) | 63 (22- 92) |
| Median performance status 1 | ( 0 – 3) |
| Previous chemotherapy | 178/212 (84%) |
Results
Clinical response to therapy
The clinical response and the 5-year percentages of survival observed in the overall patients and in relation to the single tumor histotypes are reported in Table 2. A complete response (CR) was achieved in 2/212 (1%) patients (non-small cell lung cancer: 1; gastric cancer: 1). Moreover, a partial response (PR) was obtained in other 14/212 (7%) patients (non-small cell lung cancer (NSCLC): 2; colorectal cancer: 2; pancreatic adenocarcinoma: 1; hepatocarcinoma: 1; biliary tract cancer: 2; ovarian cancer: 2; bladder cancer: 1; triple negative breast cancer (TNBC): 1; melanoma: 2). Then, an objective tumor regression was achieved in 16/212 (8%) patients. A stable disease (SD) was observed in 95/212 (45%). Therefore, a disease control (DC) (CR+PR+SD) was achieved in 111/212 (52%) patients, whereas the remaining 101 patients (48%) had a progressive disease (PD). As shown, the 5-year survival observed in the overall patients and in relation to their clinical response is illustrated in Figure 1. The 1-year, 3-year and 5- year survival percentages were 46%, 18%, and 11%, respectively. Moreover, the survival time obtained in patients, who achieved an objective tumor regression (CR+PR), was significantly longer with respect to that found in those, who had no tumor regression (P<0.01). Finally, the survival time found in patients with SD was also significantly longer than that observed in patients with PD (P<0.05).
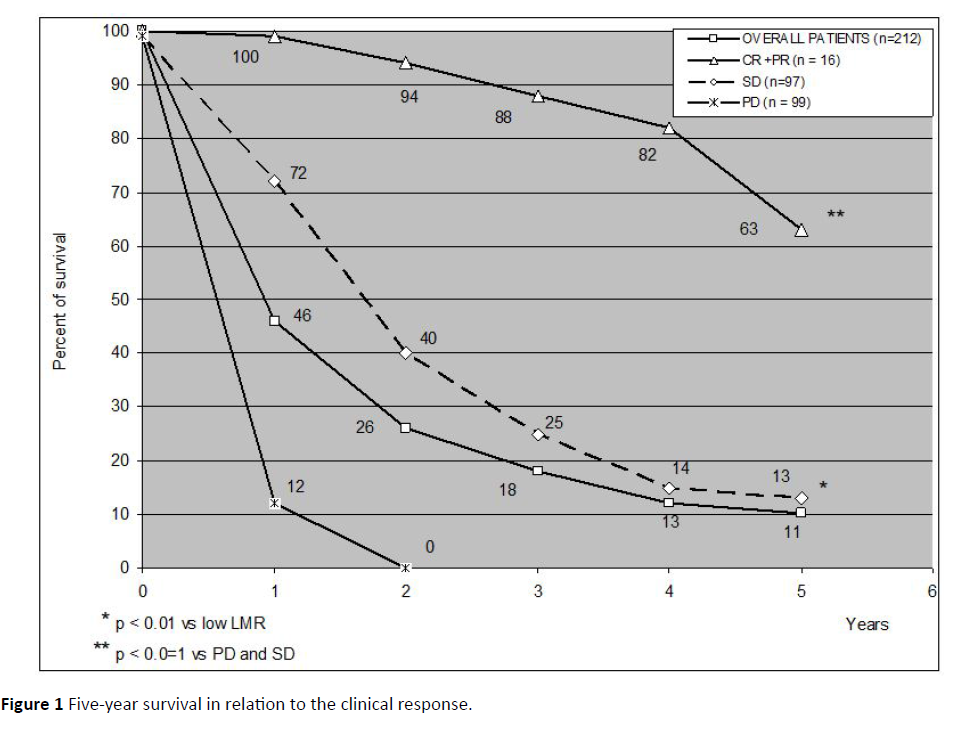
Figure 1: Five-year survival in relation to the clinical response.
Table 2 Clinical response (WHO citeria) and survival time to pineal endocrine therapy (P.E.T.) in 212 untreatable advanced cancer patients, and their relation to tumor histotype.
| Patients + | Clinical Response ++ | Survival Time (Year) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | CR | PR | CR + PR (%) | SD | DC (%) | PD | 1 | 2 | 3 | 4 | 5 | |
| Overall Patients | 212 | 2 | 14 | 16 (-8%) | 95 | 111 (-52%) | 101 | 98 (-46%) | 56 | 38 | 27 | 23 (-11%) |
| Tumor Histotype | ||||||||||||
| Lung cancer | 36 | 1 | 2 | 3 | 16 | 19 | 17 | 17 | 9 | 7 | 5 | 5 |
| -NSCLC | 29 | 1 | 2 | 3 | 14 | 17 | 12 | 15 | 7 | 6 | 4 | 4 |
| -SCLC | 7 | 0 | 0 | 0 | 2 | 2 | 5 | 2 | 2 | 1 | 1 | 1 |
| Colorectal cancer | 25 | 0 | 2 | 2 | 13 | 15 | 10 | 13 | 8 | 5 | 4 | 4 |
| Pancreatic cancer | 22 | 0 | 1 | 1 | 10 | 11 | 11 | 12 | 4 | 3 | 2 | 1 |
| Gastric cancer | 12 | 1 | 0 | 1 | 3 | 4 | 8 | 3 | 3 | 3 | 2 | 1 |
| Biliary tract cancer | 11 | 0 | 2 | 2 | 2 | 4 | 7 | 6 | 3 | 2 | 2 | 1 |
| Hepatocarcinoma | 6 | 0 | 1 | 1 | 3 | 4 | 2 | 3 | 2 | 1 | 0 | 0 |
| Ovarian cancer | 14 | 0 | 2 | 2 | 7 | 9 | 5 | 9 | 6 | 3 | 2 | 2 |
| Bladder cancer | 5 | 0 | 1 | 1 | 3 | 4 | 1 | 3 | 2 | 1 | 1 | 1 |
| Prostate cancer | 4 | 0 | 0 | 0 | 3 | 3 | 1 | 3 | 3 | 2 | 2 | 2 |
| TNBC | 6 | 0 | 1 | 1 | 2 | 3 | 3 | 3 | 2 | 2 | 1 | 1 |
| Soft tissue sarcoma | 15 | 0 | 0 | 0 | 8 | 8 | 7 | 5 | 3 | 3 | 2 | 2 |
| Melanoma | 10 | 0 | 2 | 2 | 4 | 6 | 4 | 4 | 2 | 2 | 1 | 1 |
| Glioblastoma | 46 | 0 | 0 | 0 | 21 | 21 | 25 | 19 | 9 | 4 | 3 | 2 |
NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer; TNBC: triple negative breast cancer, CR: complete response; PR: partial response; SD: stable disease; DC: disease control; PD: progressive disease
Immune effect of therapy
From the point of view of the immunological status is concerned, abnormally low pretreatments values of LMR were seen in 131/212 (62%) patients. The clinical response in relation to LMR pretreatment values are shown in Table 3. As reported, both objective tumor regression and DC percentages observed in patients with normal pretreatment values of LMR were significantly higher than those found in patients with abnormally low LMR values prior to therapy (P<0.01 and P<0.05, respectively). In addition, as illustrated in Figure 2, the 5-year percentage of survival observed in patients with normal LMR values prior to therapy was significantly longer than that achieved in patients with low pretreatment LMR values (P<0.01). Finally, as far as patients with PD are concerned, 44/101 (44%) patients, who had a PD, continued the pineal therapy despite the progression of their disease, because their improved clinical status. After 6 months and 1 year, only 34/ 101 (34%) and 2/101 (2%) were still alive. Both patients still alive at 1 year had continued the pineal therapy, whereas no patient, who interrupted the treatment, was alive. Moreover, the percentage of 9-month survival achieved in progressed patients, who continued the pineal therapy, was significantly longer than that found in those, who interrupted the endocrine treatment (14/44 (32%) vs. 0/57, P<0.05). Finally, abnormally low LMR values prior to therapy were seen in 70/101 (69%) patients with PD. The 9-month survival percentage observed in patients with PD but normal pretreatment values of LMR was significantly longer than that found in progressed patients with abnormally low values of LMR prior to therapy (9/31 (29%) vs. 5/70 (7%), P<0.05). The treatment was well tolerated, and most patients experienced a clear subjective benefit in mood, anxiety, sleep quality and asthenia. No biological toxicity occurred under pineal therapy, and some transient undesirable effects, such as headache, increase in anxiety, and sleep disturbances, occurred for few days in only 23/212 (11%) patients, without the need to interrupt the treatment.
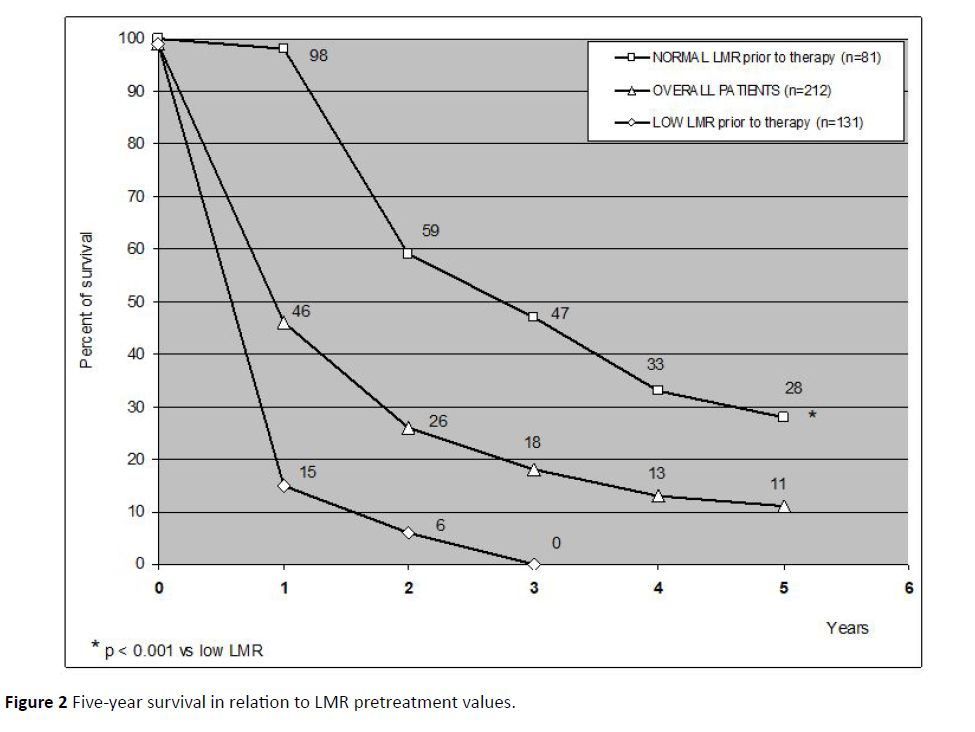
Figure 2: Five-year survival in relation to LMR pretreatment values.
Table 3 Clinical response (WHO criteria) in relation to LMR pretreatment values to pineal endocrine therapy (P.E.T.) in 212 untreatable advanced cancer patients.
| Lmr Pretreatment Values + | Clinical Response ++ | ||||||
|---|---|---|---|---|---|---|---|
| n | CR | PR | CR+PR (%) | SD | DC (%) | PD (%) | |
| Normal Values | 81 | 2 | 9 | 11 (14%) * | 52 | 63 (78%)** | 18 (22%) |
| Low Values | 131 | 0 | 5 | 5 ( 4%) | 43 | 48 (37%) | 83 (63%) |
+ LMR: lymphocyte-to-monocyte ratio; normal values more than 2.1; ++ CR: complete response; PR: partial response; SD: stable disease; DC: disease control; PD: progressive disease
*P<0.01 vs. low LMR values; P<0.05 vs. low LMR values
Discussion
According to previous preliminary clinical results [23-25], this study confirms in a greater number of untreatable advanced cancer patients and for a longer period of follow-up that the endocrine therapy with high-dose of MLT in association with the administration of the other two main anticancer molecules of the pineal gland, including 5-MTT and pinealine, may induce some tumor regression and prolong the survival time in patients eligible for the only palliative therapy because of the lack of response to the previous antitumor therapies, and life expectancy lower than 1 year. Moreover, the pineal endocrine therapy-induced prolongation of the survival time has appeared to be greater in patients, who achieved an objective tumor regression or disease stabilization, by suggesting that pineal endocrine-induced control of cancer growth is not a simple epiphenomenon, since it has been proven to predict a longer survival. This finding is not surprising since the only MLT has been already observed to represent the only molecule capable of counteracting the whole six main mechanisms responsible for cancer dissemination [23], including stress-induced immunosuppression, cancer cell transformation, intercellular joint alterations, stimulation of the neoangiogenic processes, tumor cell production of immunosuppressive factors and tumor expression of FAS-L, which allows the apoptosis of T lymphocytes after their interaction with the cancer cells [30]. In addition, the antitumor activity of MLT may be enhanced by the concomitant association with other pineal anticancer molecules, by justifying the possible evidence of tumor regressions or tumor stabilization also in very advanced cancer patients, for whom no other standard anticancer therapy may be available. Moreover, this study would suggest that the efficacy of a pineal endocrine antitumor therapy is greater in patients with normal pretreatment values of LMR, which may synthetize the whole status of the anticancer immunity in the single cancer patient [29]. The different efficacy of therapy may be influenced by the previous therapies, namely radiotherapy, because of the influence on lymphocyte count. Then, the evidence of abnormally low LMR values would reflect an immunosuppressive status of the anticancer immunity, with a consequent lower efficacy of the various anticancer treatments. Finally, previous studies had already shown a greater efficacy of the anticancer therapies in the presence of a real spiritual faith condition, as assessed by an adequate clinical test [31]. Some recent biomarkers, such as LMR, could be use full to clinically monitor the immune status of cancer patients [32,33]. Then, in the presence of a clinical response consisting of objective tumor regression or neoplastic disease stabilization, of a normal LMR values prior to therapy and an adequate spiritual faith score, it is probable that the pineal endocrine antitumor therapy may contribute to the control of the neoplastic growth and modify the prognosis of an untreatable advanced neoplastic disease also in patients, for whom no other conventional anticancer therapy may be available. On the contrary, tumor histotype does not seem to influence the efficacy of the pineal anticancer therapy in a relevant manner, even though glioblastoma and pancreatic adenocarcinoma would seem to represent the less responsible neoplasms to the treatment. However, by considering their low life expectancy after failure of the various therapies, glioblastoma and cancer of pancreas would be also influenced by the pineal therapy, at least in terms of survival time with respect to the expected one. Obviously, further randomized studies with the only best supportive care (BSC) or with BSC plus the pineal endocrine anticancer therapy will be required to confirm that the administration of the main anticancer molecules produced by the pineal gland may prolong the survival time also in patients with advanced cancer, eligible for the only palliative therapy and with life expectancy less than 1 year, since the survival of untreatable cancer patients, for whom no other standard anticancer therapy is available, is constantly generally less than 1 year or 6 months.
Conclusion
This preliminary study, by showing a possible increase in the survival time in patients with untreatable tumors and life expectancy less than 1 year, then suitable for the only supportive care by the simple administration of the immunostimulating pineal hormones would suggest that the separation between palliative and curative has to be abrogated by the existence in the nature of several non-toxic anticancer agents, namely within the same human body, which could be administered to untreatable cancer patients with respect to the only palliative therapy. Moreover, further studies by evaluating other immune parameters, such as tumor infiltrating lymphocytes, will be required to better define the immunomodulating effects of pineal therapy.