Research Article - (2017) Volume 7, Issue 5
Haroun AA1, Kamaluddeen KK2, Alhaji I, Magaji Y and Oaikhena EE
1Department of Biological Sciences, Nigerian Defence Academy, Kaduna, Nigeria
2School of Science, Environment and Technology, Abertay University, Dundee, Scotland
Corresponding Author:
Haroun AA
Department of Biological Sciences
Nigerian Defence Academy, Kaduna, Nigeria
Tel: +08034532593
E-mail: aliahmharoun@yahoo.com
Received date: August 12, 2017; Accepted date: October 04, 2017; Published date: October 13, 2017
Citation: Haroun AA, Kamaluddeen KK, Alhaji I, Magaji Y, Oaikhena EE (2017) Evaluation of Heavy Metal Tolerance Level (MIC) and Bioremediation Potentials of Pseudomonas aeruginosa Isolated from Makera-Kakuri Industrial Drain in Kaduna, Nigeria. Eur Exp Biol. Vol. 7 No. 5:28. doi: 10.21767/2248-9215.100028
Copyright: © 2017 Haroun AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Bacterial strains isolated from the Kakuri drain were characterized and subjected to various concentrations of heavy metal salts and their ability to tolerate the heavy metals (Minimum Inhibitory Concentration-MIC) was determined. This shows their ability to tolerate and survive in environments with high levels of heavy metal salts. Eight(8) heavy metals were considered , and included ; Cobalt Chloride (CoCl2), Cadmium Chloride (CdCl2),Copper Sulphate (CuSo4), Mercury Chloride (HgCl2), Nickel Chloride (NiCl2), Potassium Dichromate (K2Cr207), Lead Chloride (PbCl2) and Zinc Sulphate (ZnS04). Strains were subjected to varied millimolar concentrations (1, 2, 5, 10, 20, 30 and 50 mM). Positive and Negative controls were set up to a certain the tolerance level amongst the strains. 100% growth of all strains was observed at 1 mM concentration, while 100% growth was recorded with CuS04, ZnS04 and HgCl2. Most strains were inhibited or could not tolerate the salts at 10 mM concentration, with the exception of ZnS04, PbCl2, and CdCl2. No growth (100% inhibition) was noticed on plates with 20, 30, 40 or 50 mM concentrations. It therefore shows that the strains isolated from the kakuri drain could withstand presence of heavy metals salts up to a concentration of 10 mM.
Keywords
Bacterial strains; Toxicity; Public health
Introduction
Metals are introduced into aquatic systems as a result of the weathering of soils and rocks, from volcanic eruptions, and from a variety of human activities involving the mining, processing or use of metals or substances that contain metal contaminants [1]. Metals from human activity has the highest potential of toxicity and pollution and includes wastewaters coming from metal plating industry, automobile, electrical and electronic materials, home appliances, pipes, caps, guns, mechanics and dye industries [2]. Also mining and smelting of metal ores, industrial emissions and applications of insecticides and fertilizers have all contributed to elevated levels of heavy metals in the environment [3]. Metals are directly or indirectly involved in all aspects of microbial growth, eg Na, Ca, Mg, Cu, K, Ni, Zn, etc. Most elements however, can interact with microbial cells and be accumulated as a result of physic-chemical mechanisms and transport systems of varying specificity, independent on, or directly and indirectly dependent on metabolism [4]. Virtually all metals, including the essential metal micronutrients, are toxic to aquatic organisms as well as humans if exposure levels are sufficiently high [5]. One of the major components of industrial discharges that go untreated into the environment, are the heavy metals [6]. Metals discharged into water bodies are not biodegraded but undergoes chemical or microbial transformations, creating large impact on the environment and public health [7]. Biological removal (using Bacteria) of heavy metal contaminants from aquatic effluents offers great potential when metals are present in trace amounts. Many microbial species such as bacteria, fungi, yeast and algae are known to be capable of adsorbing heavy metals on their surfaces and/or accumulating within their structure. The detoxifying ability of these resistant microorganisms can be manipulated for bioremediation of heavy metals. Industrial effluents having heavy metals resistant can be treated with these microorganisms by the processes in the environment as biosorption, bioaccumulation and bioprecipitation.
Bacterial strains isolated from the kakuri drain in Kaduna town, Kaduna state, Nigeria were characterized using the biochemical method and further confirmed as Pseudomonas aeruginosa by molecular analysis. Literature supports the result obtained showing Pseudomonas as having high tolerance for heavy metals. The kakuri drain carries wastewater from the makera-kakuri industrial site to the river Kaduna which eventually empties into river Niger. Industries located in the layout include, textile industries, an automobile assembly plant, an arms factory with an electroplating unit, and a superphosphate fertilizer company among others. The wastewater from the industrial site containing the heavy metals among other substances is utilized for irrigation purposes in most developing countries. Vegetable crops raised with such wastewaters include among others, lettuce, cabbage, tomatoes, waterleaf, spinach etc. The removal of heavy metals from industrial effluents can be achieved by using microorganisms, and Pseudomonas species have been documented as being effective agents in bioremediation [8]. Generally, the mechanism involved in the removal of metal salts in the environment by microorganisms include; adsorption, complexation, precipitation and volatilization.
Materials and Methods
The makera-kakuri drain is one of several major drains in Kaduna metropolis. It is distinct from other drains because it carries wastewater from several industrial plants and the surrounding residential area. Located in Kaduna, capital of Kaduna state in Northern Nigeria, the drain eventually empties in to river Kaduna [9]. The state lies at latitude 10.20 N and longitude 7.23 E.
Wastewater samples were collected from designated sample collection points across the length of the drain. Five (5) sampling points were noted and samples were collected according to the procedure. Phenotypic characterization of cultured isolates from samples was conducted and include among others, oxidase, catalase, swarming motility, twitching motility, protease, NaCl2 tolerance, gelatinase activity, and swimming motility.
Minimum inhibitory concentration (MIC)
Minimum Inhibitory Concentration of the heavy metal resistant bacteria isolates grown on heavy metal incorporated media was determined by gradually increasing the concentration of the heavy metal on the growth media (KB*) each time until the strains fail to grow on the plates. MIC was noted when the isolates fail to grow on plates even after 10 days of incubation [10]. Varied Millimolar (mM) concentrations of the following heavy metal salts were prepared for the assay: Cobalt Chloride (CoCl2), Cadmium Chloride (CdCl2), Copper Sulfate (CuSo4), Mercury Chloride (HgCl2), Nickel Chloride (NiCl2), Potassium Dichromate (K2Cr2O7), Lead Chloride (PbCl2) and Zinc Sulfate (ZnSo4). The starting concentration of the heavy metals (Zn2+, Cu2+, Cd2+, Co2+, Ni2+, Cr6+, Pb2+ and Hg2+) was 1 mM. Subsequent concentrations were 2, 5, 10, 20, 30 and 50 mM.
Positive and Negative Controls were employed. A Positive control consisted of a metal deficient medium inoculated with the microorganism.
A Negative control consisted of a metal-supplemented medium without the microorganism. The drop-inoculated plates were then incubated at room temperature (20?C) for 24-48 hrs. Growth was recorded as positive, while no growth was noted as negative. Growth was represented in percentage and calculated as number of strains that tested positive over the total no of strains and control in the experiment; that is -
Gr(%)=rt/tn*100
Where Gr=growth measured in percentage; rt= no. of resistant strains and tn=total no of strains plus controls.
eg table ##ZnSO4 (10 nM)
Gr(%)=22/33 *100=66.6%, recorded as 22(67)
Tolerance to heavy metal salts was performed using salts of Copper, Lead, Zinc, Mercury, Chromium, Nickel, Cobalt and Cadmium. The minimum inhibitory concentration (MIC) was recorded for each strain at the different concentrations.
Results
Minimum inhibitory concentration for the heavy metals is calculated (Table 1 and Figure 1).
| Heavy Metal | Concentration in mM | ||||||
|---|---|---|---|---|---|---|---|
| Salt | 1 | 2 | 5 | 10 | 20 | 30 | 50 |
| CuSO4 | 33(100) | 33(100) | 29(88) | 0(0) | 0(0) | 0(0) | 0(0) |
| KCrO7 | 33(100) | 23(70) | 8(24) | 0(0) | 0(0) | 0(0) | 0(0) |
| NiCl2 | 33(100) | 31(93) | 12(36) | 0(0) | 0(0) | 0(0) | 0(0) |
| ZnSO4 | 33(100) | 33(100) | 27(82) | 22(67) | 0(0) | 0(0) | 0(0) |
| PbCl2 | 33(100) | 24(73) | 16(49) | 5(15) | 0(0) | 0(0) | 0(0) |
| CoCl2 | 33(100) | 12(36) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| CdCl2 | 33(100) | 24(73) | 5(15) | 2(6) | 0(0) | 0(0) | 0(0) |
| HgCl2 | 33(100) | 33(100) | 26(79) | 0(0) | 0(0) | 0(0) | 0(0) |
| Salt | 1 | 2 | 5 | 10 | 20 | 30 | 50 |
| CuSO4 | 100 | 100 | 88 | 0 | 0 | 0 | 0 |
| KCrO7 | 100 | 70 | 24 | 0 | 0 | 0 | 0 |
| NiCl2 | 100 | 93 | 36 | 0 | 0 | 0 | 0 |
| ZnSO4 | 100 | 100 | 82 | 67 | 0 | 0 | 0 |
| PbCl2 | 100 | 73 | 49 | 15 | 0 | 0 | 0 |
| CoCl2 | 100 | 36 | 0 | 0 | 0 | 0 | 0 |
| CdCl2 | 100 | 73 | 15 | 6 | 0 | 0 | 0 |
| HgCl2 | 100 | 100 | 79 | 0 | 0 | 0 | 0 |
Table 1: Heavy metal salts minimum inhibitory concentration (MIC).
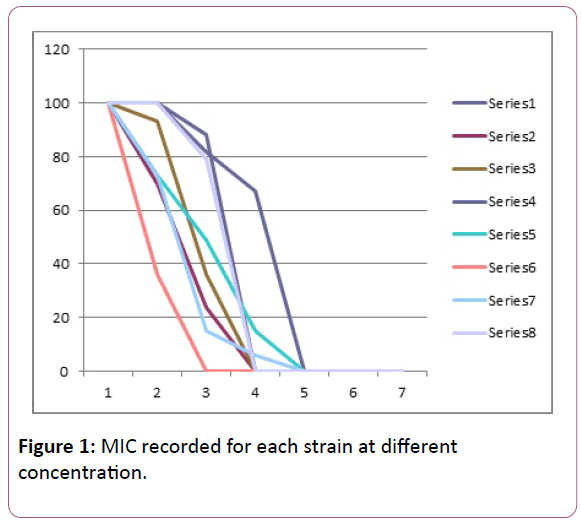
Figure 1: MIC recorded for each strain at different concentration.
Discussion
The Result shows the Minimum Inhibitory Concentration (MIC) of the various metal salts against the isolates. All the Bacteria stains reasonably tolerated low concentrations of the salts at 1 mM and 2 mM concentrations [11]. There was proliferent growth of strains at these concentrations. At 5 mM Cobalt Chloride concentration however, the growth was completely inhibited while copper sulphate was well tolerated even at 5 mM (88%). No growth was observed in copper sulphate (CuS04), Potassium Dichromate (K2Cr207), Nickel Chloride (NiCl2), Cobalt Chloride (CoCl2) and Mercury Chloride (HgCl2) at 10 mM concentration. Growth was also completely inhibited at 20 mM [12]. Subsequently no growth was observed at 30 mM and none also at 50 mM concentrations. The minimum inhibitory concentration for most isolates as observed from the experiment was therefore at 5 mM concentration. Most of the strains showed very little resistance or low tolerance to Cobalt Chloride (CoCl2). The highest degree of tolerance was observed with Zinc followed by Lead, where more than 67% of the isolates grew (showed resistance) at 10 mM concentration. There was also some degree of tolerance (resistance) towards Lead Chloride (PbCl2). The order of tolerance towards (highest degree of tolerance to least degree of tolerance) the heavy metals by the isolates could therefore be presented as follows– Zn>Pb>Cd>Cu>Hg>Ni>Cr>Co. This shows Cobalt as the least tolerated metal by the isolates followed by Chromium while Zinc was highly tolerated by the isolates with more than 50% growth noticed even at 10 mM concentration, in comparison to previous studies on metal tolerance [13]. Hence, Pseudomonas strains isolated from the industrial drain at kakuri could be important in the bioremediation of wastewater in the drain. The industrial and domestic waste from the Makera-Kakuri environs which find its way into the drains, contain levels of metals that could pose threat to health.
Recommendation
Results of the experiment showed the need to maintain the recommended level of toxic substances as laid down by the local authority. This will help keep the overall level of toxic substances low and within safe limits. The above could be achieved by enforcing the law on industries, to make sure they have standard treatment plants and hence release waste within the permissible level recommended by the authority.
Acknowledgment
Wish to acknowledge the contribution of the following Dr A Spiers, Mal Akpai Mohammed and Mrs Musili O Haroun.