Research Article - (2015) Volume 2, Issue 1
Safila Naveed*, Zehra Ashraf and Tasleem Mukhtar
Faculty of Pharmacy Jinnah University for women Karachi, Pakistan
Corresponding Author:
Safila Naveed
Faculty of Pharmacy Jinnah University for women Karachi, Pakistan
E-mail: safila117@yahoo.com
Objective: The aim of study is to perform the assay of brand and active of Pioglitazone HCL (piozer 15mg) by using UV visible spectrophotometer.
Methodology: Take brand of Hilton pharma Piogiltazone HCL (piozer 15mg) is compared with active of Piogiltazone HCL with brand (Piozer). Initially weighted 20 tablets of brand of Piogiltazone HCL (Piozer) then these tablets were uniformly grounded by using mortar and pestle and calculating the average weight of powder equivalent to 0.1006 gm of active i.e. Piogiltazone HCL was transferred into a volumetric flask containing 10mL of water. The solutions were sonication for 5-6 min and make up volume up to 50 ml with DI water.
Result: The data result shows that the absorbance is directly proportion to concentration, it obeys to Beers lambert law and assay of brand (Piozer) and active of Piogiltazone HCL are within range of USP and British pharmacopeia linearity. The correlation coefficient for active and brand of Piogiltazone HCL (PLH) were found to be 0.9998 for brand (Piozer), 0.9915 for active (PLH).
Conclusion: This method is easily used in routine Quality Control (Q.C) lab for the determination of the assay of any brand of Pioglitazone HCL by using UV spectrophotometer. This method is simple, accurate and economical.
Keywords
Pioglitazone HCL, thiazolidenediene, UV visible spectrophotometer
Introduction
Pioglitazone HCL (PLH) "( 5-[[4-[2- (5-ethylpyridin-2-yl) ethoxy] phenyl] methyl]-1, 3Thiazolidine2, 4-dione)” is existing in class of thiazolidenediene [1] and molecular mass of proglitazone HCL is 356.44 g/mol and chemical formula OF PLH is C19H20N2O3S [2]. (See Figure 1)
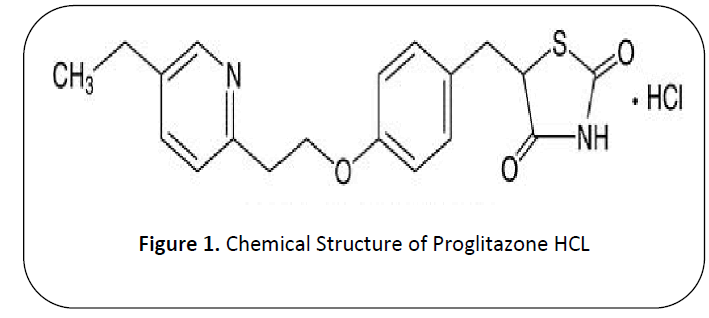
Figure 1: Chemical Structure of Proglitazone HCL
It is colorless needles from dimethylformamide and water. Melting point of Proglitazone HCL is 183-184 deg C and Dissociation Constants is pKa = 5.19 (pyridine) [3]. It is soluble in N, N-dimethyl formaimide and partially insoluble in water [4]. PLH is generally not used as a first line therapy. It alters transcription of genes, due to this it influences the carbohydrates and lipid metabolism as a result amount of protein synthesis and metabolism changes occurs [5]. PLH also reducing the circulation of insulin in a dipose tissue, muscles and glycaemia and it increases the peripheral and hepatic insulin activity by inhibition of hepatic glucogenesis, [6] it is used alone or combination with insulin or metformin, sulfonylurea. It is used in Type 2 diabetes Mellitus and decreases the chance of cardiovascular risk [7]. The pharmacokinetics of Proglitazone HCL (PLH) is plasma concentration is 2-2.5hrs, Bio Availability is 83%, and half-life is 3-7 hrs and eliminated in faces. [8] Main adverse effect of Pioglitazone HCL is highly risk to cause bladder cancer, risk of hypoglycemia and chances of anemia [9]. One of research demonstrate that the method of PLH was developed and also validate by highperformance liquid chromatography (sensitive and accurate equipment) using with ultraviolet (UV) detection at 269nm for the examination of pioglitazone [10]. An accurate method of high liquid performance chromatography method developed for analysis of pioglitazone hydrochloride in tablets by using mixture of ammonium format buffer with formic acid to pH 3 and acetonitrile and detected at 225nm [11]. The aim of study is to perform the assay of brand and active of Pioglitazone HCL (piozer 15mg, company name Hilton pharma).
Experimental Analysis
Double beam UV visible spectrophotometer has been used for the measurement of spectra of piogitazon. De- Ionized (DI) was used as a solvent for the assay of PLH.
Selection of wavelength
Pioglitazone HCL solution of 200(ppm) was prepared in DI water. Solution was scanned in the Ultraviolet (in the range of 200-400nm region). The wavelength (λ max) was observed at 220 nm and this wave length was used for absorbance measurement.
Standard stock solution
Accurately weighed 0.1006 gm of Piogiltazone HCL was transferred to a volumetric flask and adds 50ml of DI water.
Sample preparation
Take brand of Hilton pharma Piogiltazone HCL (piozer 15mg) and compare with active of Piogiltazone HCL. Brand was purchased from Pharmacy which is located in Karachi, Pakistan. Initially weighted 20 tablets of brand of Piogiltazone HCL i.e. Piozer then these tablets were uniformly ground by using mortar and pestle and calculating the average weight of powder equal to 0.1006 gm of active i.e. Piogiltazone HCL was transferred into a volumetric flask containing 10mL of water. The solutions were sonication for 5-6 min and make up volume up to 50 ml with DI water.
Procedure
Prepared standard and sample solutions and strength of solution is 200 ppm in 50 ml, check the absorbance of sample in UV visible spectrophotometry. After Preparation and check the absorbance in 1cm cell at the wavelength of maximum absorbance at 220nm, with the blank solution. Calculate the quantity (in mg) of Piogiltazone HCL per tablet.
Results and Discussion
The aim of the Research was to perform the pharmaceutical assay of Piogiltazone HCL active of Piogiltazone HCL compare with its brand i.e. Piozer by using UV spectrophotometer. Pharmaceutical company name, Brand name and absorbance of 200ppm in 50 ml detected in UV spectrophotometric method were particularly given in “tables 1, 2 and figure 2”. [14-22]
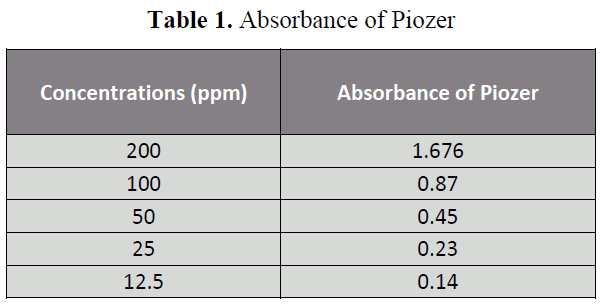
Table 1: Absorbance of Piozer
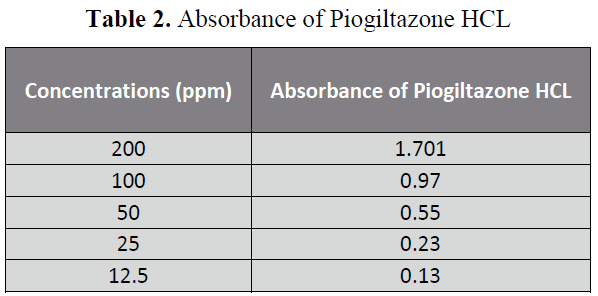
Table 2: Absorbance of Piogiltazone HCL
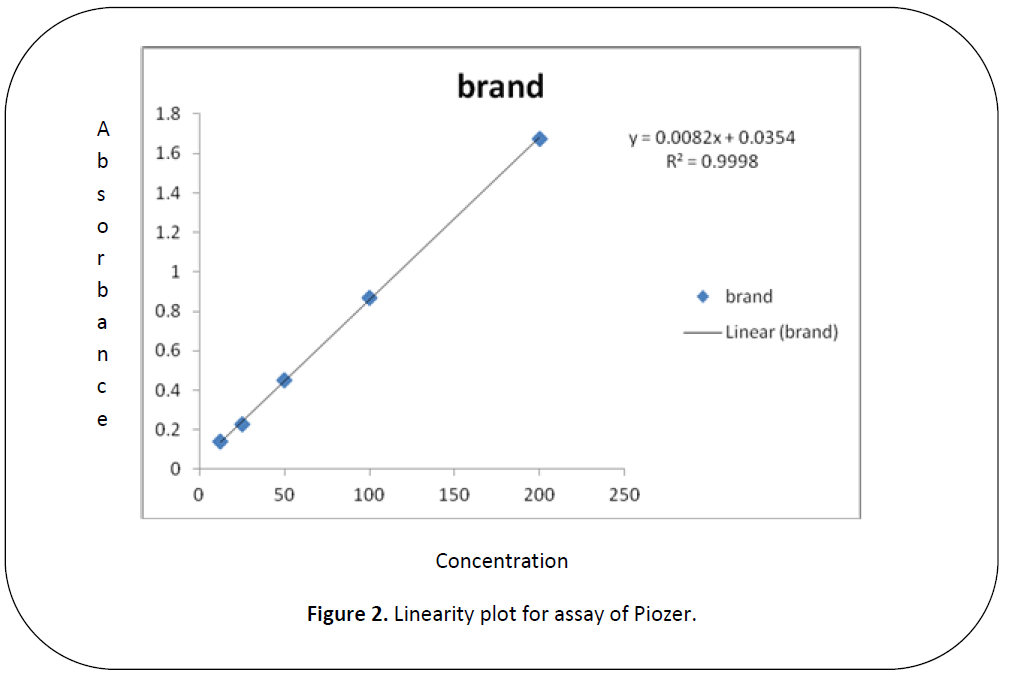
Figure 2: Linearity plot for assay of Piozer.
Five dilutions of "200(ppm), 100 (ppm), 50 (ppm), 25 (ppm), 12.5 (ppm)” for each brand and active of Piogiltazone HCL were prepared and check absorbance. The linearity of the brand and active were detected by preparing solution of "200 (ppm), 100 (ppm), 50 (ppm), 25 (ppm) and 12.5 (ppm)” [12]. The data result shows that the absorbance is directly proportion to concentration, it obeys to Beers lambert law and assay of brand and active of Piogiltazone HCL are within range of USP and British pharmacopeia linearity given in" figure 3 and 2”.We have done these types of assay for brand and active which helpful for selecting drugs. [13]
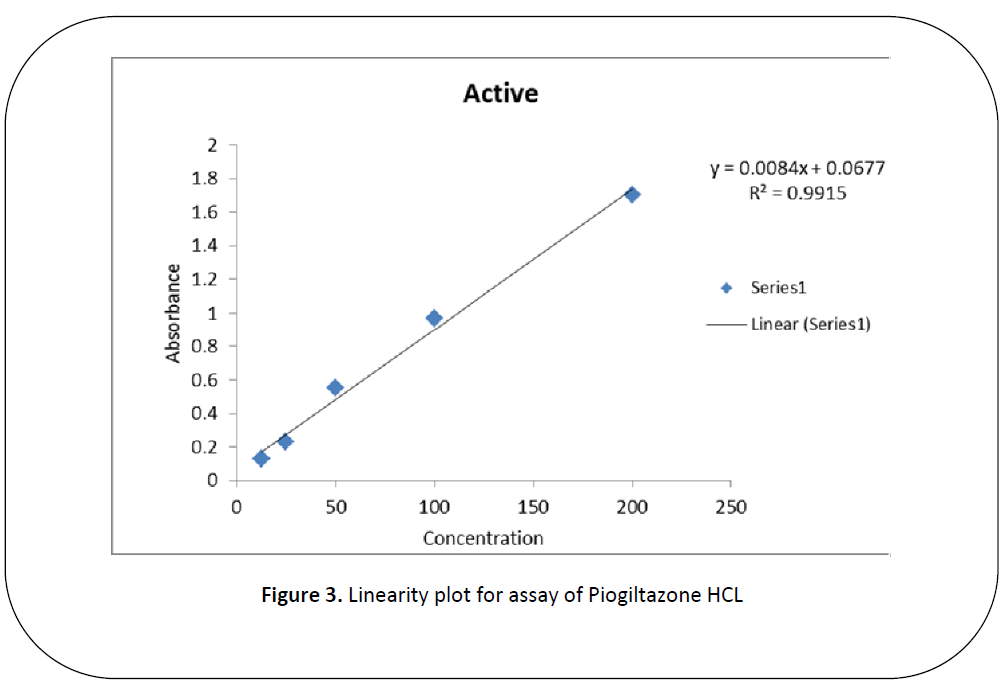
Figure 3: Linearity plot for assay of Piogiltazone HCL
Conclusions
An excellent linear relationship was observed in the concentration ranges of "200 (ppm), 100 (ppm), 50 (ppm), 25 (ppm) and 12.5 (ppm)” for active (PLH) and brands (piozer). The correlation coefficient for active and brand were found to be 0.9998 for brand (piozer), 0.9915 for active (PLH) these are within the limit.
This method is easily used in routine Quality Control (Q.C) lab for the determination of the assay of any brand of Pioglitazone HCL by using UV visible spectrophotometer. This method is simple, accurate and economical.