Research Article - (2017) Volume 7, Issue 3
Serap Sunar1* and Guleray Agar2
1Department of Pharmaceutical Botany, Faculty of Pharmacy, Erzincan University, Erzincan, Turkey
2Department of Biology, Faculty of Science, Atatürk University, Erzurum 25240, Turkey
Corresponding Author:
Serap Sunar
Department of Pharmaceutical Botany
Faculty of Pharmacy, Erzincan University, Erzincan Turkey
Tel: +90 446224 53 44
E-mail: ssunar@erzincan.edu.tr
Received Date: April 03, 2017; Accepted Date: May 09, 2017; Published Date: May 19, 2017
Citation: Sunar S, Agar G. Allelopathic Effect of Convolvulus arvensis L. Extracts on The Phytohormones and Cytological Processes of Zea mays L. Seeds. Eur Exp Biol 2017, 7:15. doi: 10.21767/2248-9215.100015
Copyright: © 2017 Sunar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The effects of different methanol extracts of Convolvulus arvensis on phytohormones (indole acetic acid (IAA), gibberellic acid (GA), abscisic acid (ABA) and salicylic acid (SA)) levels of corn were investigated. It was observed that all concentrations of methanol extracts (50 µl, 75 µl, 100 µl) of roots, stems and leaves of C. arvensis decreased the mitotic index and also caused abnormalities in chromosomes. On the other hand, phytohormone levels have been changed by application of different extracts at various concentrations. Compared with the control, GA levels decreased significantly whereas ABA levels increased in all the application groups. SA and IAA levels showed changes depending upon applied extracts and their concentrations.
Keywords
Allelopathic potential; Convolvulus arvensis; Phytohormone; Mitotic index
Introduction
Invasive species have the potential to affect the structure of native plant communities and they particularly damage to cultivated fields. Mechanisms underlying these effects of invasive species may displace natives through competition, changes in ecosystem processes, allelopathy or other mechanisms, and the relative importance of these factors remains unclear [1,2]. Allelopathy is expected to be an important mechanism in the plant invasion process because of the lack of co-evolved tolerance of resistant vegetation to new species. Chemicals produced by the invader might facilitate their rapid spread and establishment of dominance in the invaded regions.
Recently, it was reported that invasive species is (potentially important trait change structures of native plant communities). Affecting many physiological processes and the effect on ion uptake these allelochemicals reduce seed germination and seedling growth [1,3] inhibits plant growth. Thus, it was thought that allelochemicals are important factors in the success of many invasive plants for establishing virtual monoculture and may contribute to the ability of particular invasive species to become dominant in invaded plant communities [4,5].
C. arvensis L. is a perennial plant and an invasive species that belongs to the family Convolvulaceae. It is reported that C. arvensis is one of the 15 most important harmful species of the world and the species is a serious problem for 32 different crops in more than 44 countries. Based on a recent European survey, C. arvensis is one of the 15 most important weed species in 8 out of 10 main crops [6].
C. arvensis has a high light requirement and is not very competitive when shaded. It is a strong competitor during water shortages due to a deep tap root with numerous lateral roots C. arvensis. In addition, methanol extract of C. arvensis has been reported to have allelopathic on the growth and some physiological process of wheat. In this study, low concentration methanol extract of C. arvensis (75,150,300 ppm) has been observed to have a stimulatory effect on lengths and dry weights of both root and shoot. It stimulates the activities of catalase, peroxidase, phenoloxidase, and the content of chlorophyll, carbohydrate, protein and phenolic compounds [7].
Superoxide dismutase suppression affects lipid peroxidation and H2O2. But the highest concentration (600 ppm) was reported to inhibit all abovementioned parameters except lipid peroxidation and H2O2. These effects of the methanol extract have been linked to the phenolic content such as the abundance of p-coumaric, p hydroxybenzoic acids, traces of resorcinol, cinnamic acids from methanol extract of C. arvensis. However the effects on physiological and cytological processes of methanol extract of C. arvensis have not been studied yet [8,9]. This study aims to investigate the effects of methanol extracts of root, stem and leaf of this plant on mitotic index and phytohormone levels of corn seedlings.
Materials and Methods
Plant material
C. arvensis was collected in the flowering stage in July 2010 from areas near Atatürk University, Erzurum Turkey. A reference specimen is deposited in the ATA herbarium (Department of Biology, Atatürk University, Erzurum, Turkey). The collected plants were dried in shade; leaves and roots were separated from the stem and all of them ground through a 2 mm mesh. Methanol is a common solvent for allelochemical analyses, because it extracts both polar and non-polar compounds, thus most potential phytotoxins are extracted. The dried and powdered leaves, roots and stems (approximately 500 g) were extracted with 1 litre of methanol using a Soxhlet extractor for 72 hours. The extract was filtered using filter paper and then concentrated in a vacuum at -40°C using a rotary evaporator. The residues obtained were stored in a freezer at -80°C for further tests.
Germination conditions
Corn seeds were surface sterilized with 2.5% (w/v) NaOCl for 3 min, washed with sterile distilled water and paper- blotted. The seeds were soaked in sterile distilled water for 1 hour, and then for 15 hours (for corn) and 30 hours (for redroot pigweed). Seeds were germinated in 15 cm diameter Petri dishes on two layers of sterile Whatman No. 1 filter paper. The plant extracts were diluted with deionized water to prepare the concentrations of 50, 75 and 100%, and 10 ml extract was added to each Petri germination plate. The controls were treated only with distilled water [10,11]. The Petri dishes were placed in a growth chamber (25°C, 70% humidity and continuously dark). When the roots length reaches 1.5-3.0 cm, suitable for cytological preparations, they were collected and fixed in acetic alcohol (1:3). Analyses of phytohormones were conducted on germinated seeds 8 days after incubation.
Cytological preparations
Cytological preparations were made by the Feulgen’s squash technique. Different slides for each treatment were examined to determine the effects of plant extracts on the mitotic index (MI) of each. The MI was calculated as the ratio of the number of dividing cells to the total number of cells [11]. Chromosomal aberrations (CA) were counted in the different mitotic stages (abnormal mitoses) and interphase stage. Mutagenic effect was estimated as the percentage of cells with bridges and fragments.
The total number of mitotic aberrations was calculated as % of aberrant cells=Total number of mitotic aberrations/Total number of dividing cells × 100.
Phytohormones
Salicylic acid and Indole-3-acetic acid (IAA) were prepared as described by Kuraishi et al. and Turker et al. One gram of germinated and frozen seeds was powdered in liquid nitrogen. The cold methanol was added to the fine powder and stored at 4°C for 24 hrs in the dark. The powder was homogenized in an Ultra Tissue Lysis (Ultrasonic Processor, Jenway Ltd. Essex, UK) and filtered through a filter paper (Whatman No. 1). Then the filtrates were collected [12]. The residue was reprocessed in the same way as described above and combined with the former one to minimize the loss of phytohormones. The filtrates were filtered through poly-tetrafluoroethylene (PTFE) filters (0.45 μm). Methanol was removed at 35°C under reduced pressure. The extracts were redissolved in K2PO4 buffer (pH 8.5) and centrifuged at 10.000 g for 1 hour at 4°C. Then the supernatants were put in flask (25 cm3), each containing 1g poly vinyl poly pyrrolidone (PVPP, Sigma Chemical Co. UK.), well mixed and filtered through filter paper (Whatman No. 1). The filtrates were introduced to Sep-Pak C18 cartridges (Waters, Hichrom Ltd. UK). The hormones were adsorbed by cartridges and remnants were removed. The hormones were eluted from the cartridges with 80% methanol and collected in vials. The hormone extracts were injected into HPLC to detect IAA [13].
The analysis of gibberellic acid equivalents was done as described by Fujioka et al. and Cakmak et al. with some modifications. The frozen tissue samples (1 g) were ground in liquid nitrogen and homogenized in 3 ml methanol. The homogenate was mixed with 80% methanol, kept overnight at 4°C and then filtered. The residue was re-extracted with 80% methanol for 4 hours, re-filtered and combined with the former supernatant [14]. Methanol was removed under reduced pressure at 35°C. The aqueous residue was adjusted to pH 2.5 (2N HCl). This solution was then partitioned with 5% (m/v) sodium bicarbonate and separated gibberellic acid equivalents were injected into HPLC.
The isocratic system was used for HPLC analysis. The extracts in the vials were injected into HPLC equipped with a Waters 6000A pump (Waters, Hichrom LTD. UK); Ultraviolet detector (Unicam Analytical Systems, Cambridge, UK) and μBondapak C18 column (Waters, Hichrom Ltd. UK) using acetonitrile (12.00%; pH 4.98) as the mobile phase. The flow rate, pressure and wavelength were selected to 2 ml min-1, 2000 psi and 265 nm, respectively [15,16]. Under these conditions, the retention time of GA3, SA and IAA were determined as 2.85, 3.88 and 7.17 minutes for standards, respectively. GA3 was used as standard solvent for determining Glyco- gambogic acid like compounds (GAs).
The analysis of abscisic acid was done as described by Unyayar et al. with some modifications. The freeze-dried tissue sample (1 g) was ground in liquid nitrogen and homogenized in 3 cm3 of 100% methanol. The homogenate was stirred at 4°C for 24 hrs in darkness. Then PVPP (0.5 g) was added to homogenate and thoroughly mixed. The mixture was filtered through filter paper (Whatman No. 1) and methanol was removed under reduced pressure at 35°C. The residue was dissolved with 1.5 ml of 0.5 M potassium dihydrogen phosphate (KH2PO4) buffer (pH 8.3). The combined organic phases were partitioned three times against hexane and three times against ethyl acetate at pH 3. The ethyl acetate of the combined organic fractions was removed under reduced pressure at 35°C. The residue was dissolved in 100% methanol and loaded onto Bondasil DEA (Waters Hichrom) column. After the column was washed with 100% methanol, absorbed hormone was eluted with methanol containing 0.5% acetic acid and collected in vial [17,18]. The hormone extracts were injected into a high performance liquid chromatography system (HPLC) to detect applied behavioral analysis (ABA) levels.
Statistical analysis
Results were expressed as means of different experiments ± standard deviation of triplicates. Analysis of variance was conducted using one-way ANOVA test using SPSS for Microsoft windows and means were compared by Duncan test at the 0.01 level of confidence [19].
Results
All methanol extracts (except for 75 and 100 μl leaf extract for corn) significantly (P ≤ 0.01) inhibited the GA level of each treatments compared with the control. The highest and lowest GA level was observed in 50 μl leaf and 100 μl leaf extract for corn [20]. On the other hand all methanol extracts significantly (P ≤ 0.01) increased ABA level of corn compared with the control (Table 1 and Figure 1).
| E.C.R (%) | GA (ng/g) | ABA (ng/g) | ||||
| Root E. | Stem E. | Leaf E. | Root E. | Stem E. | Leaf E. | |
| Control | 2004.7 ± 2.64 | 2004.7 ± 2.64 | 2004.7 ± 2.64 | 7.06 ± 1.15 | 7.06 ± 1.15 | 7.06 ± 1.15 |
| 50 µl | 1451.53 ± 1.52* | 1668.98 ± 2.08* | 1912.4 ± 2.05* | 11.35 ± 0.57 | 47.05 ± 4.04* | 10.05 ± 1.15 |
| 75 µl | 1264.01 ± 2.30* | 1424.76 ± 1.52* | 1149.95 ± 3.04* | 9.9125 ± 1.45297* | 40.53 ± 2.88* | 52.3 ± 4.61 |
| 100 µl | 1144.29 ± 1.73* | 1100.89 ± 3.00* | 741.48 ± 3.04* | 8.7 ± 0.57 | 35.62 ± 2.48* | 97.80 ± 4.95* |
| *Represent significant difference over control at P≤0. 01. | ||||||
Table 1: The effect of C. arvensis extracts on GA and ABA levels on corn.
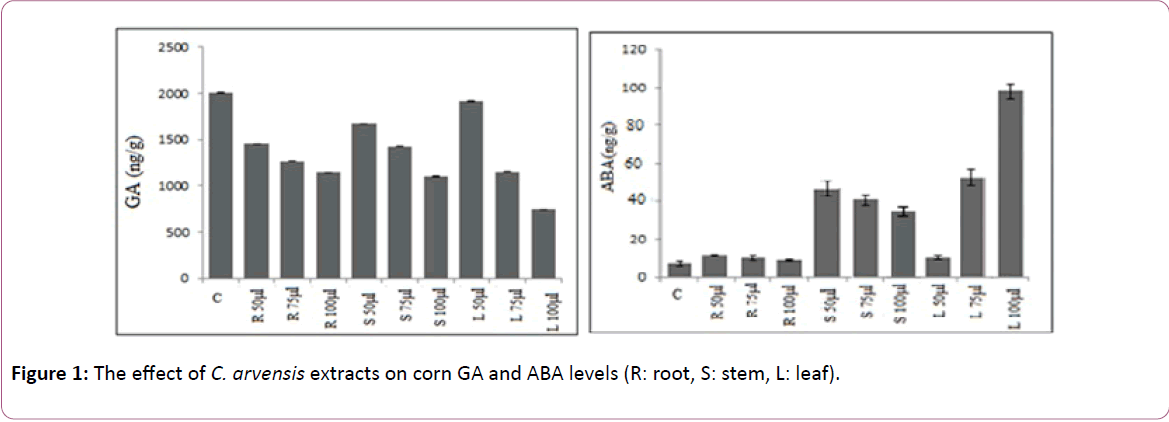
Figure 1: The effect of C. arvensis extracts on corn GA and ABA levels (R: root, S: stem, L: leaf).
The highest level of ABA was observed in the application of leaf extract (especially 75 and 100 μl) for corn (Figure 1). The statistically significant increase was determined in SA level depending on the various concentration of leaf and root extract for corn seed (Table 2 and Figure 2) [21]. The lowest level of IAA was found in the treatment of 100 μl leaf extract for corn. 50 μl extracts of C. arvensis had no important (P ≤ 0.01) inhibitory effects of IAA level on corn seed (Table 3 and Figure 2).
| Extracts | Concentration (µl) | Average | |||
| 0 (a) | 50 (b) | 75 (c) | 100 (d) | ||
| Stem | 7.6 ± 0.1a | 6.8 ± 0.1b,a | 6.4 ± 0.1c,a | 5.6 ± 0.1d,b | 6.6 ± 0.2a |
| Leaf | 7.6 ± 0.1a | 6.3 ± 0.1b,b | 6.2 ± 0.1bc,a | 6.0 ± 0.1c,a | 6.5 ± 0.2a |
| Root | 7.6 ± 0.1a | 4.6 ± 0.2b,c | 4.4 ± 0.1bc,b | 4.1 ± 0.0c | 5.2 ± 0.0ab |
| Average | 7.6 ± 0.0a | 5.9 ± 0.3b | 5.7 ± 0.3c | 5.2 ± 0.3d | |
| *Represent significant difference over control at P≤0.01. | |||||
Table 2: The effect of C. arvensis extracts on mitotic index of corn.
| E.C.R (%) | SA (ng/g) | IAA (ng/g) | ||||
| Root E. | Stem E. | Leaf E. | Root E. | Stem E. | Leaf E. | |
| Control | 260 ± 2.88 | 260 ± 2.88 | 260 ± 2.88 | 32.31 ± 1.15 | 32.31 ± 1.15 | 32.31 ± 1.15 |
| 50µl | 841.94 ± 2.08* | 1414.75 ± 1.52* | 1245.11 ± 2.64* | 14.05 ± 0.57* | 30.21 ± 0.88* | 24.96 ± 0.57* |
| 75 µl | 890.5 ± 2.64* | 1209.19 ± 3.05* | 794.99 ± 1.15* | 15.6 ± 0.57* | 20.06 ± 0.57* | 17.78 ± 0.57* |
| 100 µl | 989.86 ± 3.50* | 683.53 ± 1.73* | 502.89 ± 1.85* | 17.9125 ± 0.57* | 18.24 ± 0.57* | 13.72 ± 0.57* |
| *Represent significant difference over control at P≤0.01. | ||||||
Table 3: The effect of C. arvensis extracts on SA and IAA levels on corn.
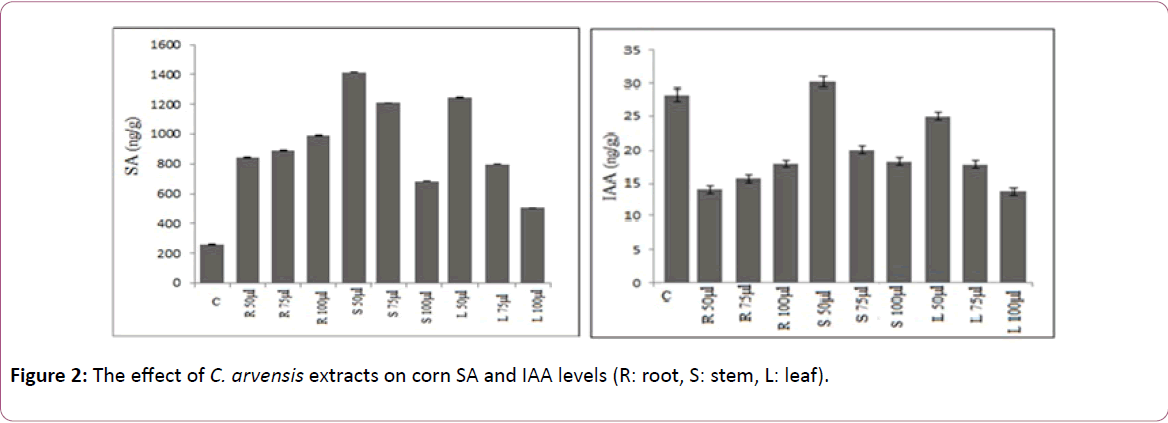
Figure 2: The effect of C. arvensis extracts on corn SA and IAA levels (R: root, S: stem, L: leaf).
On other hand, after the treatments, a number of chromosomal aberrations were observed in Zea mays in root cells (Table 4). It is clear that C. arvensis extracts decreased mitotic activity when compared with the control. The lowest mitotic activity was observed from 100 μl root extracts while the highest was 50 μl stem extract (Table 2). Chromosome abnormalities are an issue related to mitotic activity. Almost all the application revealed higher chromosome abnormalities when compared with the control [22]. The most injurious effect was produced by C. arvensis leaf extracts. The highest and lowest chromosome abnormalities level was observed in 100 μl leaf and 50 μl root extract for corn (Table 4). Data obtained from mitotic index and chromosomal abnormalities showed significant difference when the applications were compared with the control (≤ 0.01).
| Extracts | Concentration (µl) | Average | |||
| 0 (a) | 50 (b) | 75 (c) | 100 (d) | ||
| Stem | 0.0 ± 0.00d | 0.3 ± 0.03c,ab | 0.3 ± 0.03b | 0.4 ± 0.03a,b | 0.2 ± 0.04b |
| Leaf | 0.0 ± 0.00c | 0.4 ± 0.06b,a | 0.5 ± 0.06ab | 0.6 ± 0.06a | 0.4 ± 0.07a |
| Root | 0.0 ± 0.00d | 0.2 ± 0.06c,b | 0.4 ± 0.06b | 0.5 ± 0.03ab | 0.3 ± 0.06b |
| Average | 0.0 ± 0.00d | 0.3 ± 0.04c | 0.4 ± 0.04b | 0.5 ± 0.04a | |
| *Represent significant difference over control at P≤0. 01 | |||||
Table 4: The effect of C. arvensis extracts on chromosomal aberation of corn.
Discussion
Our results have showed that methanol extracts of C. arvensis decreased mitotic index, GAs and IAA level and increased ABA and SA levels in corn. The degree of these effects was mostly dependent on the extract types and the concentration of the extract tested. GAs and auxins (of which one is IAA) stimulate growth in plants. These phytohormones are synthesized in unstressed conditions and their effects cause growth events in plants. In contrast, ABA is growth inhibitory and it has been well known as “stress hormone” in plants. It means that the synthesis of ABA increases especially during stress conditions in the leaves, and it moves to all plant parts to inhibit growth and protect the plant against to the biotic and abiotic stresses. SA also has a protective effect against plant pathogens [22,23] and some abiotic stress, like ABA. In this study, determining of increment of ABA and SA levels shows that methanol extracts of C. arvensis caused stress (allelopathic stress) in corn seedlings. That is why we think that the levels of phytohormones stimulating growth (GA and IAA) decreased whereas ABA and SA synthesis increased in the seedlings. It has been reported that ABA and SA encourage activity of alternative respiration pathway [24] which is related to prevent the formation of reactive oxygen species (ROS) in plants [25]. Stress conditions cause an increase in the ROS levels and oxidative stress. In this situation, it is possible to say that ABA and SA synthesis increases to reduce oxidative damage in plants. It has been well known that exogenic ABA lead to synthesis of some new protein types, inhibition of some proteins and also increase and decrease of synthesis of some proteins which synthesize under unstressed conditions in plant cells [21,23]. It means that ABA may play a role change of the gene expression model in plants. Putting the results together, we may assume that methanol extracts of C. arvensis have a negative allelopathic affect in corn seedlings. Because of the stress, the synthesis of ABA and SA increase, while GA and IAA levels decrease to protect against to damages of the allelopathic stress in corn seedlings. From the results, we may infer that the increment of the ABA levels and SA are indicator parameters for negative allelopathic stress in plants [26,27].
Differential effects of the methanol extracts of C. arvensis on the levels of phytohormones and cytological processes of the corn tested may be due to different phenolic compounds in plant extracts. In previous studies, phenolic compounds such as p-coumaric and p hydroxybenzoic acids, resorcinol, cinnamic acids, have been isolated from the methanol extract of C. arvensis [28]. In addition, different studies have reported that phenolic compounds such as caffeic, ferulic, protocatechuic acid and vanillic acids have allelopathic effect. In particular, these studies have shown that at low concentration these phenolic compounds have stimulated the growth, anti-oxidant enzymes activity, protein and chlorophyll contents of some crops, whereas they have inhibitory effect on these parameter when they are at high concentrations. Sunar et al. showed that methanol extracts of C. arvensis had protein activity changes and genotoxic activity with random amplified polymorphic DNA (RAPD) analysis. But methanol extracts of C. arvensis on a mitotic index, and phytohormones levels have not been reported. Previous studies have also shown that various plant extracts and residues are changed by phytohormone activity in some crops [29]. The interactions between phytohormones and compounds found in plant extracts may be explained by an increase and decreases in phytohormones levels. This interaction causes changes in plant metabolism, which was evaluated by determining the contents of phytohormones and response of mitotic activity and chromosomal abnormality. The exact role of phytohormones under stress conditions is still unclear and molecular mechanisms explaining the role of phytohormones in plant stress tolerance needs to be elucidated [27].
Consequently, methanol extracts of C. arvensis have allelopathic potential in corn. Mechanisms underlying this effect may be the role of phytohormones and their mutagenic effect. Even if some of phytohormones levels increased (ABA and SA), some of them decreased (GA and IAA) under the allelopathic stress, we think that these changes are a part of the defence system against the stress. The seedlings had tried to resist, but other parts of its defence system (for example antioxidant system) were not enough for resistance to occur, and mitotic activity and chromosomal abnormalities occurred. Rowe et al. [18] has reported that DNA damage-induced increase in intracellular ROS levels is a generalized stress response that is likely to function in various signalling pathways. From our results, the corn seedlings do not have a strong defence system against to allelopathic substances in the extracts of C. arvensis, despite of the changes of phytohormone levels. The allelopathic stress may cause oxidative damages in corn cells and then indirectly cause these abnormalities.
This potential may contribute to the success of C. arvensis as an invader of new area, but field trials are needed to examine the relative importance of allelopathy as against other factors (like reactive oxygen species) and their effects on nucleic acids.
Acknowledgments
The authors thank Dr. Rahmi Dumlup?nar for the interpretation of phytohormones.